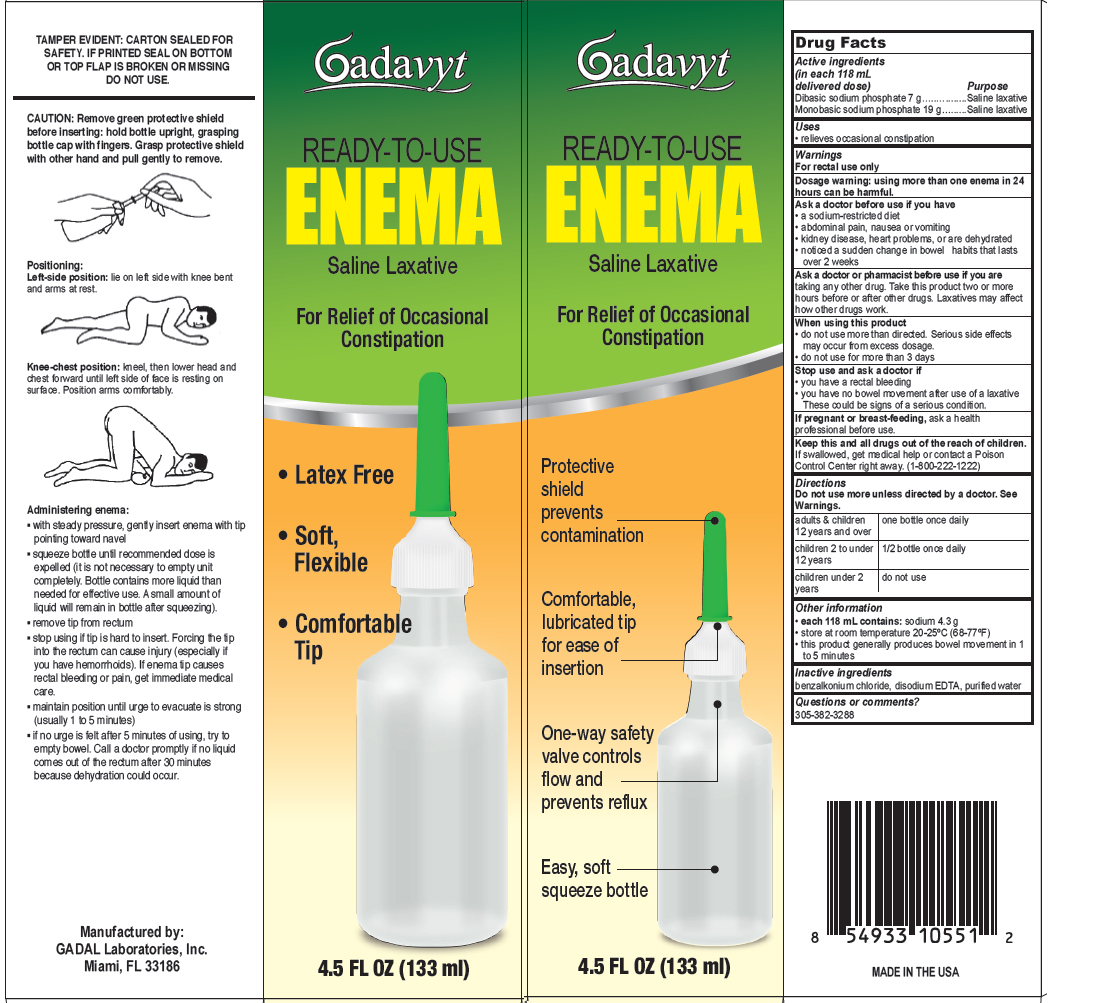
Dibasic sodium phosphate 7 g............. Saline Laxative
Monobasic sodium phosphate 19 g......... Saline Laxative
For rectal use only
Dosage warning: Using more than one enema in 24 hours can be harmful.
Ask a doctor before use if you have
- a sodium-restricted diet
- abdominal pain, nausea or vomiting
- kidney disease, heart problems, or are dehydrated
- noticed a sudden change in bowel habits that lasts over 2 weeks
taking any other drug. Take this product two or more hours before or after other drugs. Laxatives may affect how other drugs work.
When using this product- do not use more than directed. Serious side effects may occur from excess dosage.
- do not use for more than 3 days
- you have rectal bleeding
- you have no bowel movement after use of a laxative
These could be signs of a serious condition.
If swallowed, get medical help or contact a Poison Control Center right away. (1-800-222-1222)
Directions
Do not use more unless directed by a doctor. See Warnings.
| adults and children 12 years and over | one bottle once daily |
| children 2 to under 12 years | 1/2 bottle once daily |
| children under 2 years | do not use |