MUCINEX FAST-MAX DAY TIME SEVERE COLD AND MUCINEX FAST-MAX NIGHT TIME COLD AND FLU MAXIMUM STRENGTH- acetaminophen, dextromethorphan hydrobromide, diphenhydramine hydrochloride, guaifenesin, and phenylephrine hydrochloride
RB Health (US) LLC
----------
Mucinex® Fast-Max
®Day Time Severe Cold and Mucinex® Fast-Max
®Night Time Cold and Flu
Maximum Strength
Uses
- temporarily relieves these common cold and flu symptoms:
- nasal congestion
- cough (Mucinex FAST-MAX DAY TIME Severe Cold only)
- minor aches and pains
- headache
- sore throat
- runny nose and sneezing (Mucinex FAST-MAX NIGHT TIME Cold & Flu only)
- helps loosen phlegm (mucus) and thin bronchial secretions to rid the bronchial passageways of bothersome mucus and make coughs more productive (Mucinex FAST-MAX DAY TIME Severe Cold only)
- temporarily reduces fever
Warnings
Liver warning
This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 12 caplets in 24 hours, which is the maximum daily amount
- with other drugs that contain acetaminophen
- 3 or more alcoholic drinks daily while using this product
Sore throat warning
if sore throat is severe, persists for more than 2 days, is accompanied or followed by fever, headache, rash, nausea, or vomiting, consult a doctor promptly.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- with any other drug containing diphenhydramine, even one used on the skin (Mucinex FAST-MAX NIGHT TIME Cold & Flu only)
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
- for children under 12 years of age
Ask a doctor before use if you have
- liver disease
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- glaucoma (Mucinex FAST-MAX NIGHT TIME Cold & Flu only)
- a breathing problem such as emphysema or chronic bronchitis (Mucinex FAST-MAX NIGHT TIME Cold & Flu only)
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema (Mucinex FAST-MAX DAY TIME Severe Cold only)
- cough that occurs with too much phlegm (mucus) (Mucinex FAST-MAX DAY TIME Severe Cold only)
Ask a doctor or pharmacist before use if you are
- taking the blood thinning drug warfarin
- taking sedatives or tranquilizers (Mucinex FAST-MAX NIGHT TIME Cold & Flu only)
When using this product
- do not use more than directed
- excitability may occur, especially in children (Mucinex FAST-MAX NIGHT TIME Cold & Flu only)
- marked drowsiness may occur (Mucinex FAST-MAX NIGHT TIME Cold & Flu only)
- alcohol, sedatives, and tranquilizers may increase drowsiness (Mucinex FAST-MAX NIGHT TIME Cold & Flu only)
- avoid alcoholic drinks (Mucinex FAST-MAX NIGHT TIME Cold & Flu only)
- be careful when driving a motor vehicle or operating machinery (Mucinex FAST-MAX NIGHT TIME Cold & Flu only)
Stop use and ask a doctor if
- nervousness, dizziness, or sleeplessness occur
- pain, nasal congestion, or cough gets worse, or lasts more than 7 days
- fever gets worse, or lasts more than 3 days
- redness or swelling is present
- new symptoms occur
- cough comes back, or occurs with fever, rash, or headache that lasts. These could be a sign of a serious condition. (Mucinex FAST-MAX DAY TIME Severe Cold only)
Directions
- do not take more than directed (see Overdose warning)
- do not take more than 12 caplets in any 24-hour period
- adults and children 12 years of age and older: take 2 caplets every 4 hours
- children under 12 years of age: do not use
Inactive ingredients
(Mucinex FAST-MAX DAY TIME Severe Cold)
croscarmellose sodium, crospovidone, FD&C Red #40 aluminum lake, FD&C Yellow #6 aluminum lake, magnesium stearate, microcrystalline cellulose, polyethylene glycol 3350, polyvinyl alcohol, povidone, talc, titanium dioxide
Inactive ingredients
(Mucinex FAST-MAX NIGHT TIME Cold & Flu)
corn starch, croscarmellose sodium, crospovidone, FD&C Blue #1 aluminum lake, hypromellose, magnesium stearate, microcrystalline cellulose, polydextrose, polyethylene glycol, povidone, silicon dioxide, stearic acid, titanium dioxide, triacetin
Dist. by: Reckitt Benckiser
Parsippany, NJ 07054-0224
Made in England (Mucinex FAST-MAX DAY TIME Severe Cold)
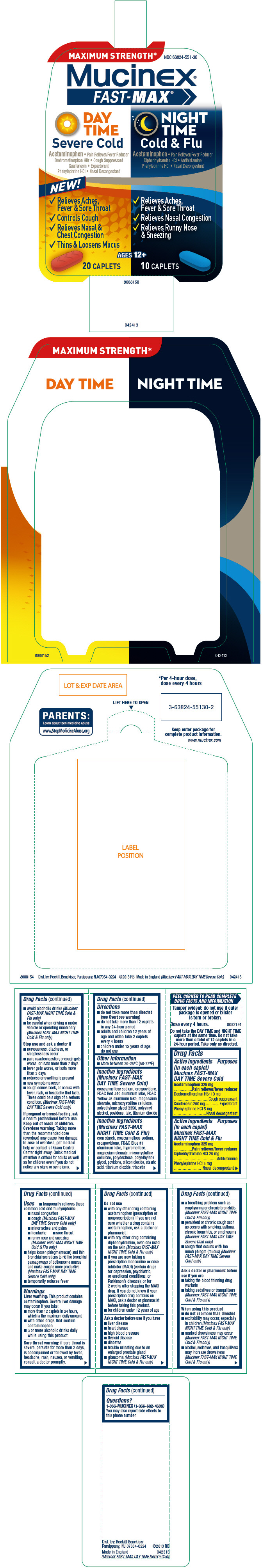
PRINCIPAL DISPLAY PANEL - Kit Carton
MAXIMUM STRENGTH*
DAY TIME
NIGHT TIME
MAXIMUM STRENGTH*
NDC 63824-551-30
Mucinex®
FAST-MAX ®
DAY
TIME
Severe Cold
Acetaminophen •Pain Reliever/Fever Reducer
Dextromethorphan HBr
•Cough Suppressant
Guaifenesin
•Expectorant
Phenylephrine HCl
•Nasal Decongestant
NEW!
-
Relieves Aches,
Fever & Sore Throat - Controls Cough
-
Relieves Nasal &
Chest Congestion - Thins & Loosens Mucus
AGES 12+
20 CAPLETS
NIGHT
TIME
Cold & Flu
Acetaminophen •Pain Reliever/Fever Reducer
Diphenhydramine HCl
•Antihistamine
Phenylephrine HCl
•Nasal Decongestant
-
Relieves Aches,
Fever & Sore Throat - Relieves Nasal Congestion
-
Relieves Runny Nose
& Sneezing
10 CAPLETS
| MUCINEX FAST-MAX DAY TIME SEVERE COLD AND MUCINEX FAST-MAX NIGHT TIME COLD AND FLU
MAXIMUM STRENGTH
acetaminophen, dextromethorphan hydrobromide, diphenhydramine hydrochloride, guaifenesin, and phenylephrine hydrochloride kit |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - RB Health (US) LLC (081049410) |