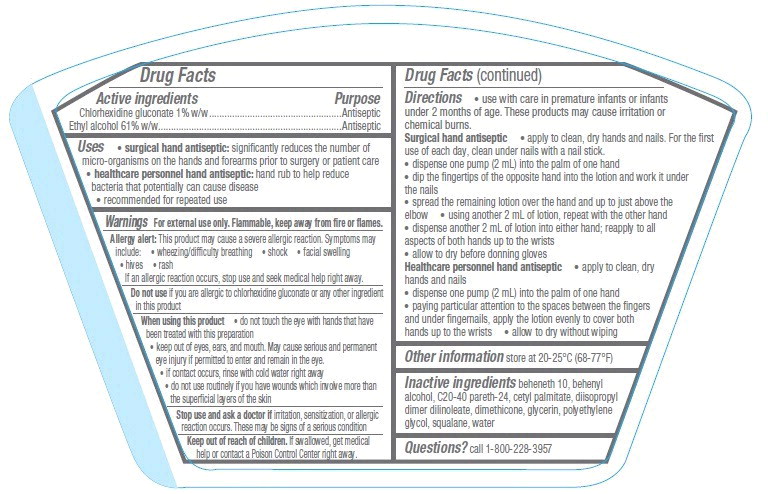
Uses
- surgical hand antiseptic: significantly reduces the number of micro-organisms on the hands and forearms prior to surgery or patient care
- healthcare personnel hand antiseptic: hand rub to help reduce bacteria that potentially can cause disease
- recommended for repeated use
Warnings
For external use only. Flammable, keep away from fire or flames.
Allergy alert: This product may cause a severe allergic reaction. Symptoms may include:
- wheezing/difficulty breathing
- shock
- facial swelling
- hives
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
When using this product
- do not touch the eye with hands that have been treated with this preparation
- keep out of eyes, ears, and mouth. May cause serious and permanent eye injury if permitted to enter and remain in the eye.
- if contact occurs, rinse with cold water right away
- do not use routinely if you have wounds which involve more than the superficial layers of the skin
Directions
- use with care in premature infants or infants under 2 months of age. These products may cause irritation or chemical burns.
Surgical hand antiseptic
- apply to clean, dry hands and nails. For the first use of each day, clean under nails with a nail stick.
- dispense one pump (2 mL) into the palm of one hand
- dip the fingertips of the opposite hand into the lotion and work it under the nails
- spread the remaining lotion over the hand and up to just above the elbow
- using another 2 mL of lotion, repeat with the other hand
- dispense another 2 mL of lotion into either hand; reapply to all aspects of both hands up to the wrists
- allow to dry before donning gloves
Healthcare personnel hand antiseptic
- apply to clean, dry hands and nails
- dispense one pump (2 mL) into the palm of one hand
- paying particular attention to the spaces between the fingers and under fingernails, apply the lotion evenly to cover both hands up to the wrists
- allow to dry without wiping
Inactive ingredients
beheneth 10, behenyl alcohol, C20-40 pareth-24, cetyl palmitate, diisopropyl dimer dilinoleate, dimethicone, glycerin, polyethylene glycol, squalane, water
Principle Display Panel – 500mL Bottle Label
3M
NDC 17518-051-01
Avagard™
(Chlorhexidine Gluconate 1% Solution and Ethyl Alcohol 61% w/w)
Surgical and Healthcare Personnel Hand Antiseptic with Moisturizers
Peel to read Drug Facts Panel before use
Made in U.S.A. for 3M Health Care
2510 Conway Ave., St. Paul, MN 55144
1-800-228-3957
www.3M.com/infectionprevention
© 2017, 3M. All rights reserved. The shape and colors of the bottle and wall bracket are trademarks of 3M.
Patent: 3M.com/Patents
MAL10110039XC
Flammable, keep away from fire or flame, heat, sparks and sources of static discharge.
LOT 0000000000
EXP 0000-00-00
16 fl oz • 500 mL
REF 9200