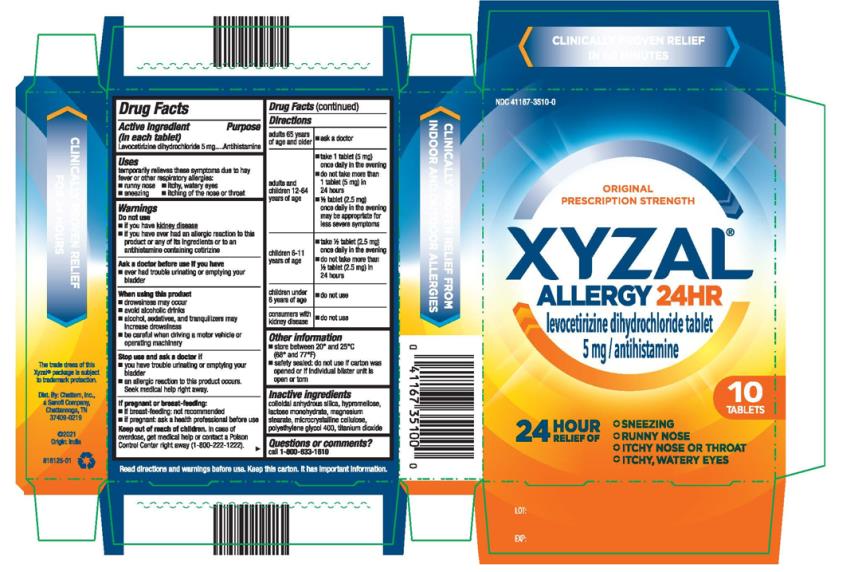
Uses
temporarily relieves these symptoms due to hay fever or other respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
Warnings
Do not use
- if you have kidney disease
- if you have ever had an allergic reaction to this product or any of its ingredients or to an antihistamine containing cetirizine
When using this product
- drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Stop use and ask a doctor if
- you have trouble urinating or emptying your bladder
- an allergic reaction to this product occurs. Seek medical help right away.
Directions
| adults 65 years of age and older | ■ ask a doctor |
| adults and children 12-64 years of age | ■ take 1 tablet (5 mg) once daily in the evening ■ do not take more than 1 tablet (5 mg) in 24 hours ■ ½ tablet (2.5 mg) once daily in the evening may be appropriate for less severe symptoms |
| children 6-11 years of age | ■ take ½ tablet (2.5 mg) once daily in the evening ■ do not take more than ½ tablet (2.5 mg) in 24 hours |
| children under 6 years of age | ■ do not use |
| consumers with kidney disease | ■ do not use |
(Note: Age ranges are bolded in the draft container labeling for tablet bottle)
Other information
- store between 20° and 25°C (68° and 77°F)
- (if blister) safety sealed: do not use if carton was opened or if individual blister unit is open or torn
(if bottled) safety sealed: do not use if carton was opened or if printed foil inner seal on bottle is torn or missing
(if stretch card) safety sealed: do not use if sealed package was torn or opened, or if printed foil inner seal on bottle is torn or missing