Keep Out of Reach of Children
If swallowed, get medical help or contact a Poison Control Center immediately. Do not induce vomiting before contacting medical help or a Poison Control Center.
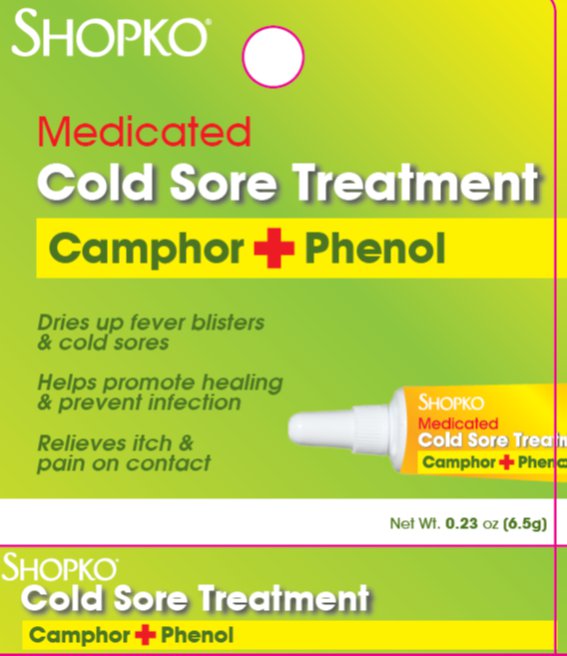
Uses
For the temporary relief of pain and itching associated with cold sores and fever blisters. First Aid to help prevent infection.
Warnings
For external use only.
Do not use over large areas of the body or with a bandage. Ask a doctor before use if you have a deep puncture wound, animal bites, or serious burns.
When using this product: do not use near the eyes. If contact occurs, rinse eyes thoroughly with water and obtain medical attention.
Stop use and consult a doctor if: conditions worsen or symptoms persist more than 7 days, or clears up and returns again in a few days.