PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
Rx only
Lidocaine Ointment USP, 5%
Laminate Tube with Child-Resistant Cap
FOR TOPICAL USE ONLY. DO NOT USE IN THE EYES
NET WT 30 g

Rx only
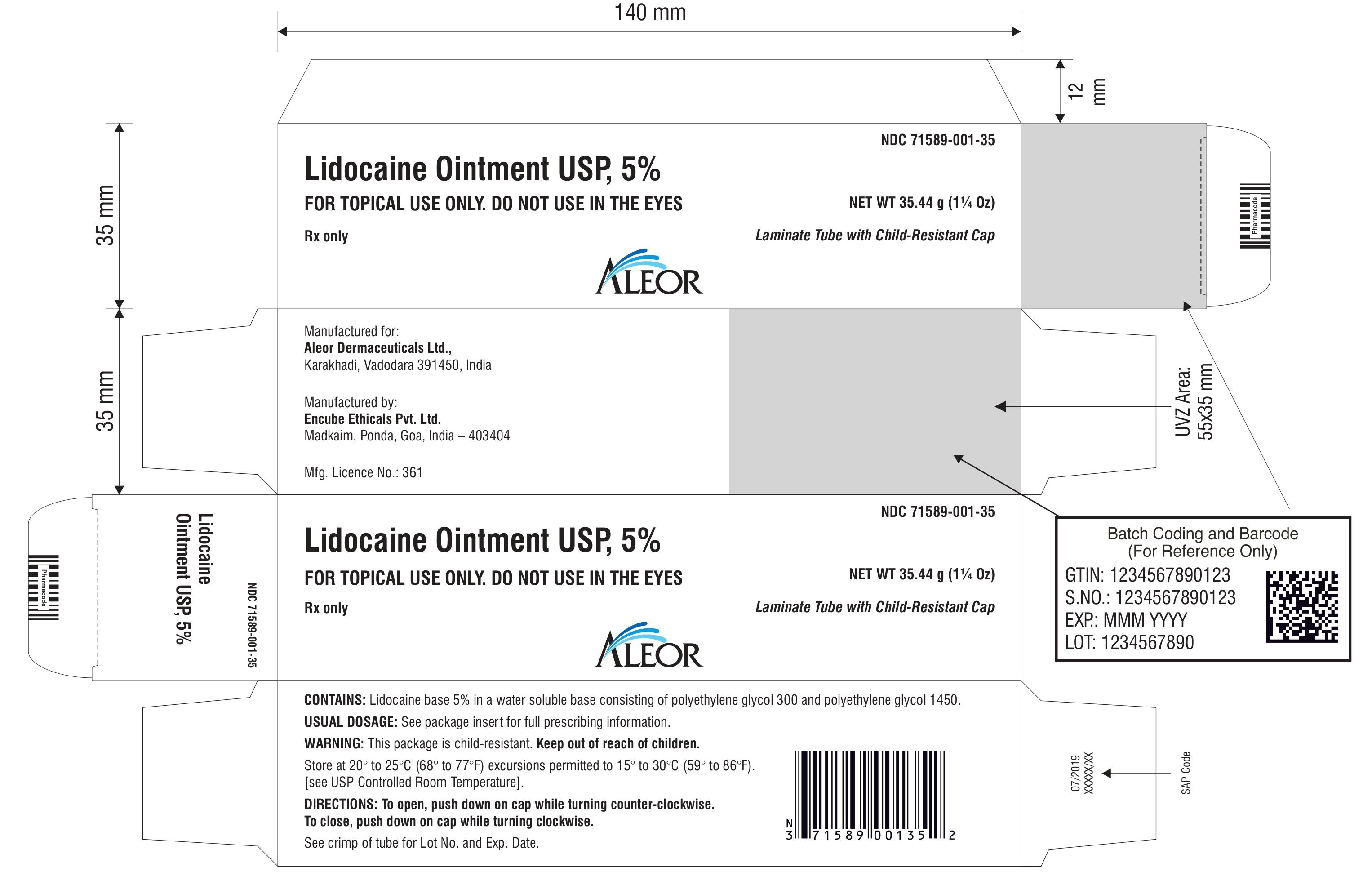
Lidocaine Ointment USP, 5%
Laminate Tube with Child-Resistant Cap
FOR TOPICAL USE ONLY. DO NOT USE IN THE EYES
NET WT 35.44 g (1¼ Oz)
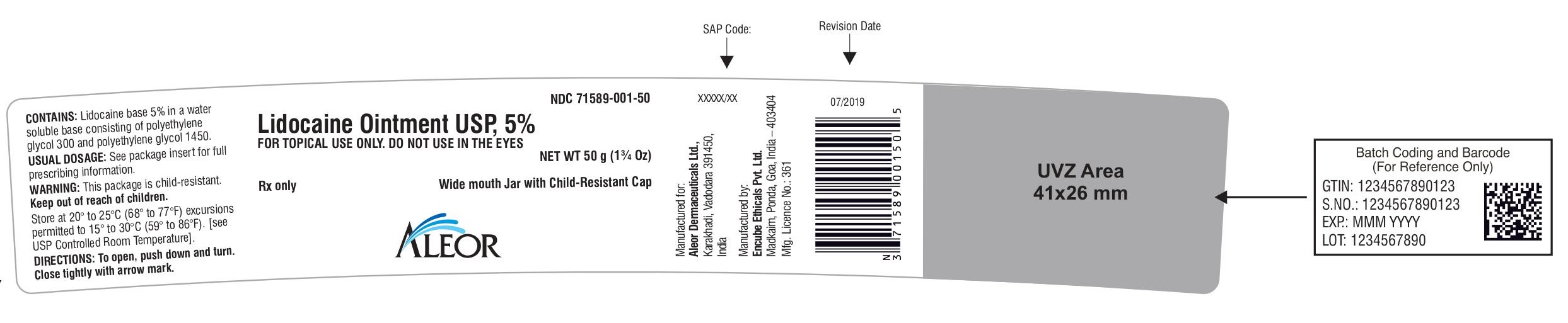
Rx only
Lidocaine Ointment USP, 5%
Wide mouth Jar with Child-Resistant Cap
FOR TOPICAL USE ONLY. DO NOT USE IN THE EYES
NET WT 50 g (1¾ Oz)