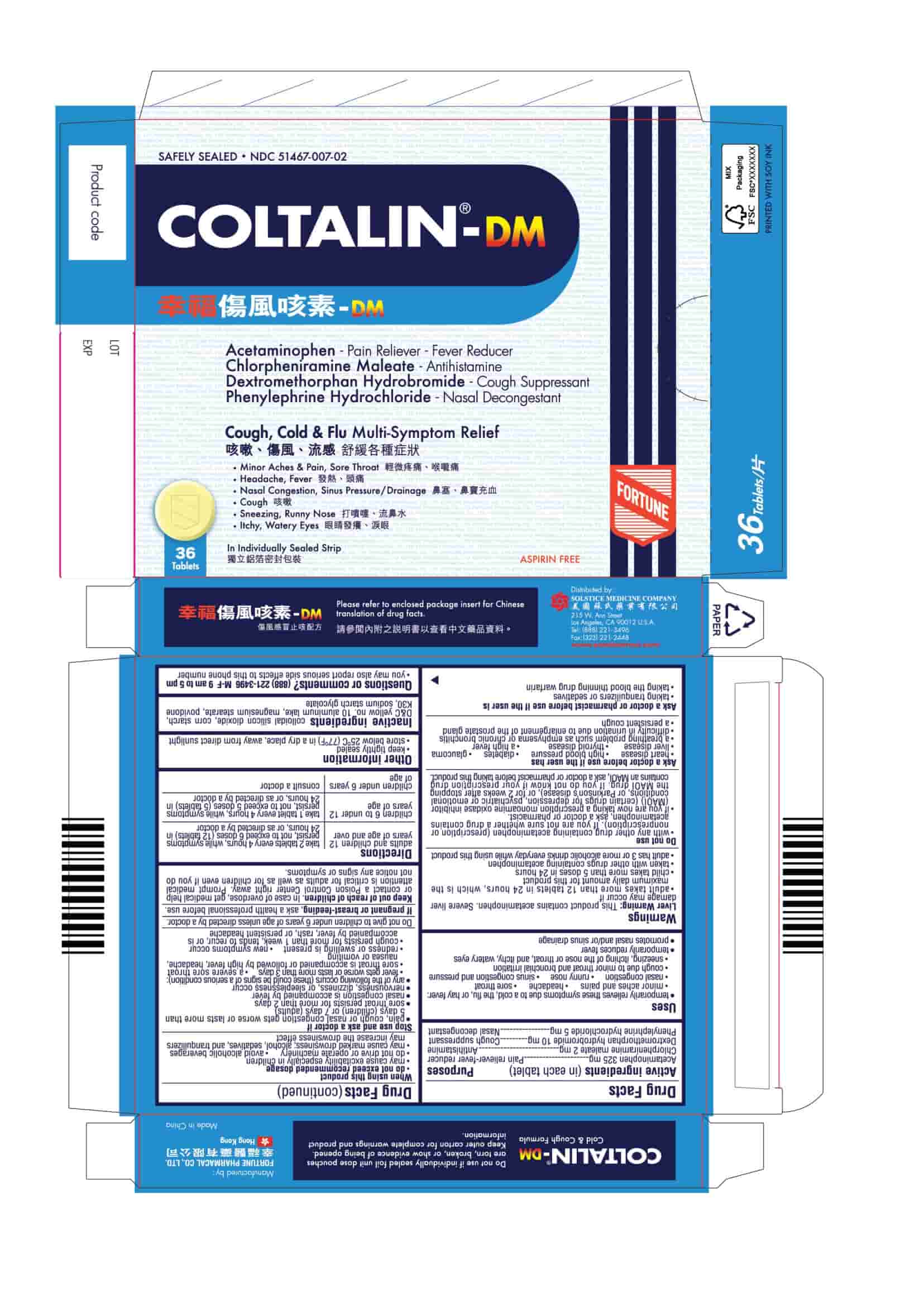
Active ingredients (in each tablet)
Acetaminophen 325 mg
Chlorpheniramine maleate 2 mg
Dextromethorphan hydrobromide 10 mg
Phenylephrine hydrochloride 5 mg
Uses
■ temporarily relieves these symptoms due to a cold, the flu, or hay fever:
■ minor aches and pains
■ headache
■ sore throat
■ nasal congestion
■ runny nose
■ sinus congestion and pressure
■ cough due to minor throat and bronchial irritation
■ sneezing, itching of the nose or throat, and itchy, watery eyes due to hay fever
■ temporarily reduces fever
■ promotes nasal and/or sinus drainage
Warnings
Liver Warning: This product contains acetaminophen. Severe liver damage may occur if
■ adult takes more than 12 tablets in 24 hours, which is the maximum daily amount for this product
■ child takes more than 5 doses in 24 hours
■ taken with other drugs containing acetaminophen
■ adult has 3 or more alcoholic drinks everyday while using this product
Do not use
■ with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
■ if the user is now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if the user has
■ heart disease
■ high blood pressure
■ diabetes
■ glaucoma
■ liver disease
■ thyroid disease
■ a high fever
■ a breathing problem such as emphysema or chronic bronchitis
■ difficulty in urination due to enlargement of the prostate gland
■ a persistent cough
Ask a doctor or pharmacist before use if the user is
■ taking sedatives or tranquilizers
■ taking the blood thinning drug warfarin
When using this product
■ do not exceed recommended dosage
■ may cause excitability especially in children
■ do not drive or operate machinery
■ avoid alcoholic beverages
■ may cause marked drowsiness; alcohol, sedatives, and tranquilizers may increase the drowsiness effect
Stop use and ask a doctor if
■ pain or nasal congestion gets worse or lasts more than 5 days (children) or 7 days (adults)
■ sore throat persists for more than 2 days
■ nasal congestion is accompanied by fever
■ nervousness, dizziness, or sleeplessness occur
■ any of the following occurs (these could be signs of a serious condition):
■ fever gets worse or lasts more than 3 days
■ a severe sore throat
■ sore throat is accompanied or followed by fever, headache, rash, nausea or vomiting
■ redness or swelling is present
■ new symptoms occur
■ cough persists for more than 1 week, tends to recur, or is accompanied by fever rash, or persistent headache
Do not give to children under 6 years of age unless directed by a doctor.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Directions
■ adults and children 12 years of age and over: 2 tablets every 4 hours, while symptoms persist, not to exceed 6 doses (12 tablets) in 24 hours, or as directed by a doctor
■ children 6 to under 12 years of age: 1 tablet every 4 hours, while symptoms persist, not to exceed 5 doses (5 tablets) in 24 hours, or as directed by a doctor
■ children under 6 of age : consult a doctor
Other information
■ keep tightly sealed
■ store between below 25 C (77 F) in a dry place, away from direct sunlight
Inactive ingredients colloidal silicon dioxide, corn starch, D&C yellow no. 10 aluminum lake, magnesium stearate, povidone K30, sodium starch glycolate
Questions or comments? (888) 221-3496 M-F 9 am to 5 pm
■ you may also report serious side effects to this phone number
COLTALIN-DM
Safely Sealed
NDC 51467-007-01
Acetaminophen - Pain Reliever-Fever Reducer
Chlorpheniramine Maleate - Antihistamine
Dextromethorphan Hydrobromide - Cough Suppressant
Phenylephrine Hydrochloride - Nasal Decongestant
Cough, Cold & Flu Multi-Symptom Relief
■ Minor Aches & Pain, Sore Throat
■ Headache, Fever
■ Nasal Congestion, Sinus Pressure/Drainage
■ Cough
■ Sneezing, Runny Nose
■ Itchy, Watery Eyes
24 Tablets
In Individually Sealed Strip
Aspirin Free

COLTALIN-DM
Safely Sealed
NDC 51467-007-02
Acetaminophen - Pain Reliever-Fever Reducer
Chlorpheniramine Maleate - Antihistamine
Dextromethorphan Hydrobromide - Cough Suppressant
Phenylephrine Hydrochloride - Nasal Decongestant
Cough, Cold & Flu Multi-Symptom Relief
■ Minor Aches & Pain, Sore Throat
■ Headache, Fever
■ Nasal Congestion, Sinus Pressure/Drainage
■ Cough
■ Sneezing, Runny Nose
■ Itchy, Watery Eyes
36 Tablets
In Individually Sealed Strip
Aspirin Free