BZK
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
BZK
Other information
- store at room temperature 15 0 to 30 0 C (5 0 - 86 0 F)
- do not reuse towelette
Aypanal
Uses
- temporarily relieves minor aches and pains due to the common cold and headache
- temporarily reduces fever
Aypanal
Warnings
Liver Warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg in 24 hours, which is the maximum daily amount.
- with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If skin reaction occurs, stop use and seek medical help right away
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription).
- If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Stop use and ask a doctor if
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- new symptoms occur
- redness or swelling is present
Keep out of reach of children.
Keep out of reach of children.
Overdose warning: In case ofl overdose, get medical help or contact a Poison Control Center right away (1-800-222-1222). Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Aypanal
Directions
- do not take more than directed (see overdose warning)
- adults and children 12 years of age and over: Take 2 tablets with water every 6 hours while symptoms last.
- do not take any more than 8 tablets in 24 hours.
- children under 12: consult a doctor
Aypanal
Other information
- store at room temperature 15 0 -30 0 C (59 0 -86 0 F)
- TAMPER EVIDENT- DO NOT USE IF OPEN OR TORN
Aypanal
Inactive igredients
microcrystalline cellulose, povidone, sodium starch glycolate, starch, stearic acid
FABC
Warnings
For external use only
Do not use
- in or near the eyes
- if you are allergic to any of the ingredients
- lin large areas of the body, particularly over raw surfaces or blistered areas
- for more than 10 days
FABC
Directions
- adults and children 2 years of age and older:
- clean the affected area
- apply a small amount of this product (equal to the surface area of the tip of a finger) onto affected area 1 to 3 times daily
- may be covered with a sterile bandage
- children under 2 years of age: consult a doctor
FABC
Inactive ingredients
aloe barbadensis juice, cetyl alcohol, diazolidinyl urea, edetate disodium, glycerin, glyceryl stearate SE, methylparaben, mineral oil, PEG-100, propylene glycol, propylparaben, stearic acid, trolamine, water
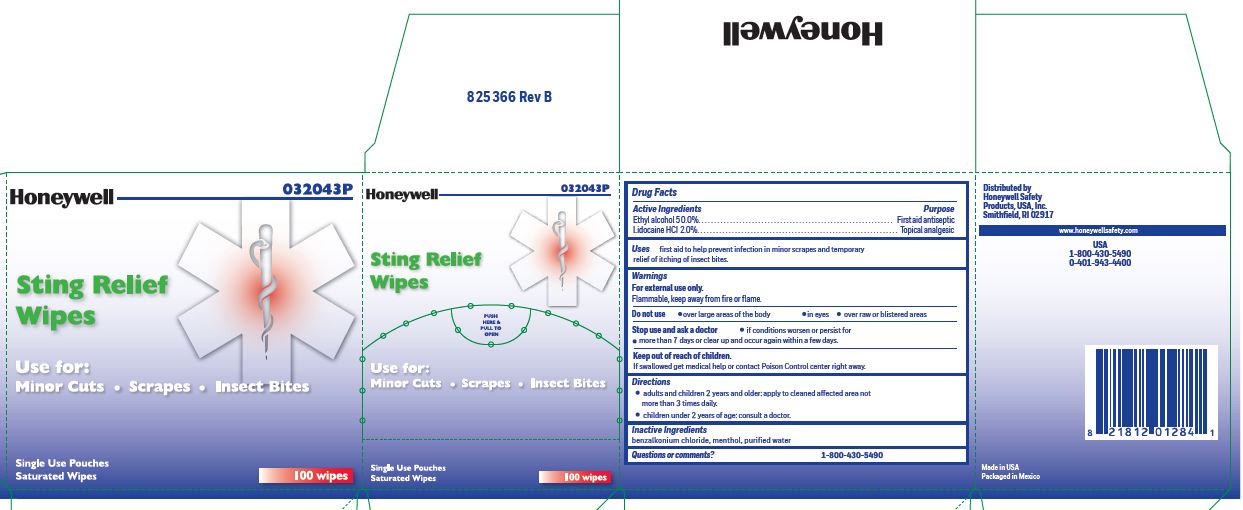
Sting Rellief
Uses
- prevent infection in minor scrapes, and temporary relief of itching of insect bites
Sting Relief
Warnings
For external use only
Flammable, keep away from open fire or flame
Sting Relief
Directions
- adults and children 2 years and older: Apply to cleaned affected area not more than 3 times daily.
- children under 2 years of age: consult a doctor.
4378
Z631580000 KIT CONTENTS
1 INSTANT COLD PACK 4" X 6"
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 TWEEZER PLASTICS 4"
1 FIRST AID GUIDE ASHI
1 ABD COMBINE PAD 5" X 9"
1 CPR FILTERSHIELD 77-100
1 FIRST AID BURN CREAM 0.9 GRM PKT 20
1 SCISSOR BDGE 4" RED PLS HDL
1 FANNY PACK RED FAK LOGO EMPTY
1 LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
6 BZK ANTISEPTIC WIPE, BULK
1 1 PR LRG NITRILE GLVES ZIP BAG
1 1" X 3" PLASTIC BANDS 16/BAG
5 SAFETEC STING RELIEF WIPES BULK
1 TRI BNDG NON WOVEN 40"X40"X56"
3 GAUZE PADS 3"X3" 12PLY
2 HEAVY FLEX LARGE PATCH 2" X 3"
2 AYPANAL EXTRA BULK 2/PK