ANTIBACTERIAL SUNBLOSSOM- triclosan soap
CVS Pharmacy
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
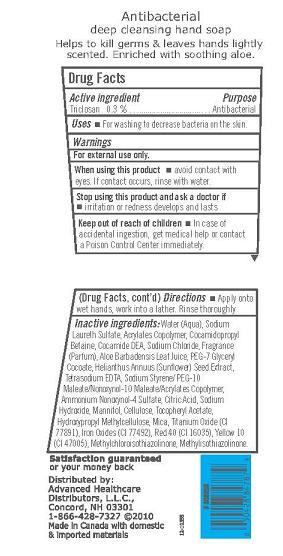
Active Ingredient
Triclosan 0.3%
Warnings
For external use only.
When using this product
- Avoid contact with eyes. If contact occurs, rinse with water.
Stop using this product and ask doctor if
- Irritation or redness develops and lasts.
Keep out of reach of children
In case of accidental ingestion, get medical help or contact a Poison Control Center immediately.
Uses
For washing to decrease bacteria on the skin.
Directions
Apply onto wet hands, work into a lather. Rinse thoroughly.
Inactive Ingredients
Water (Aqua), Sodium Laureth Sulfate, Acrylates Copolymer, Cocamidopropyl Betaine, Cocamide DEA, Sodium Chloride, Fragrance (Parfum), Aloe Barbadensis Leaf Juice, PEG-7 Glyceryl Cocoate, Helianthus Annuus (Sunflower) Seed Extract, Tetrasodium EDTA, Sodium Styrene/PEG-10 Maleate/Nonoxynol-10 Maleate/ Acrylates Copolymer, Ammonium Nonoxynol-4 Sulfate, Citric Acid, Sodium Hydroxide, Mannitol, Cellulose, Tocopheryl Acetate, Hydroxypropyl Methylcellulose, Mica,
Titanium Oxide (CI 77891), Yellow 10 (CI 47005), Red 40 (CI 16035), Iron Oxides ( CI 77492), Methylchloroisothiazolinone, Methylisothiazolinone.
PACKAGE FRONT AND BACK LABELS
- 8.5 OZ Front Panel Label: eobf.jpg
- 8.5 OZ Back Panel Label: eobb.jpg