Eyewash
Active ingredient
Sterile Water 99%
Eyewash
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyewash
Warnings
For external use only Obtain immediate medical treatment for all open wounds in or near eyes. To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Stop use and ask a doctor if
- you experience eye pain
- changes in vision
- continued redness or irritation of the eye
- condition worsens or persists
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away.
Eyewash
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
Eyewash
Inactive ingredients
sodium chloride, sodium phosphate dibasic, sodium phosphate monobasic
Eyewash
Questions
1-800-430-5490
Aspirin
Active ingredient (in each tablet)
Aspirin 325 mg (NSAID)* *nonsteroidal anti-inflammatory drug
Aspirin
Purpose
Pain reliever/fever reducer
Aspirin
Uses
temporarily reduces fever and relieves minor aches and pains associated with:
- a cold
- headache
- toothache
- muscular aches
- backache
- minor pain of arthritis
- premenstrual and menstrual periods
Aspirin
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:are:
- age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Do not use
- if you are allergic to aspirin or any other pain reliever/fever reducer
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis or kidney disease
- you are taking a diuretic
- you have asthma
Ask a doctor or pharmacist before use if you are
- taking a prescription drug for diabetes, gout or arthritis
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- ringing in the ears or loss of hearing occurs
- any new symptoms appear
If pregnant or breast-feeding,
If pregnant or breat-feeding, ask a health professional before use. It is especially important not to use aspirin during the last three months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Keep out of reach of children.
- In case of overdose, get medical help or contact Poison Control Center right away.
Aspirin
Directions
- drink a full glass of water with each dose
- adults and children 12 years of age and older: take 1 or 2 tablets every 4 hours while symptoms last, not more than 12 tablets in 24 hours
- children under 12 years of age: consult a doctor
Aspirin
Other information
- store at room temperature 15° - 30°C (59° - 86°F)
- TAMPER EVIDIENT PACKETS
- DO NOT USE IF OPEN OR TORN
Aspirin
Inactive ingredients
corn starch, croscarmellose sodium*, hypromellose*, microcrystalline cellulose*, mineral oil*, polyethylene glycol*, povidone, propylene glycol, silicon dioxide, stearic acid*, titanium dioxide*
*may contain these ingredients
Aspirin
Questions or Comments
1-800-430-5490
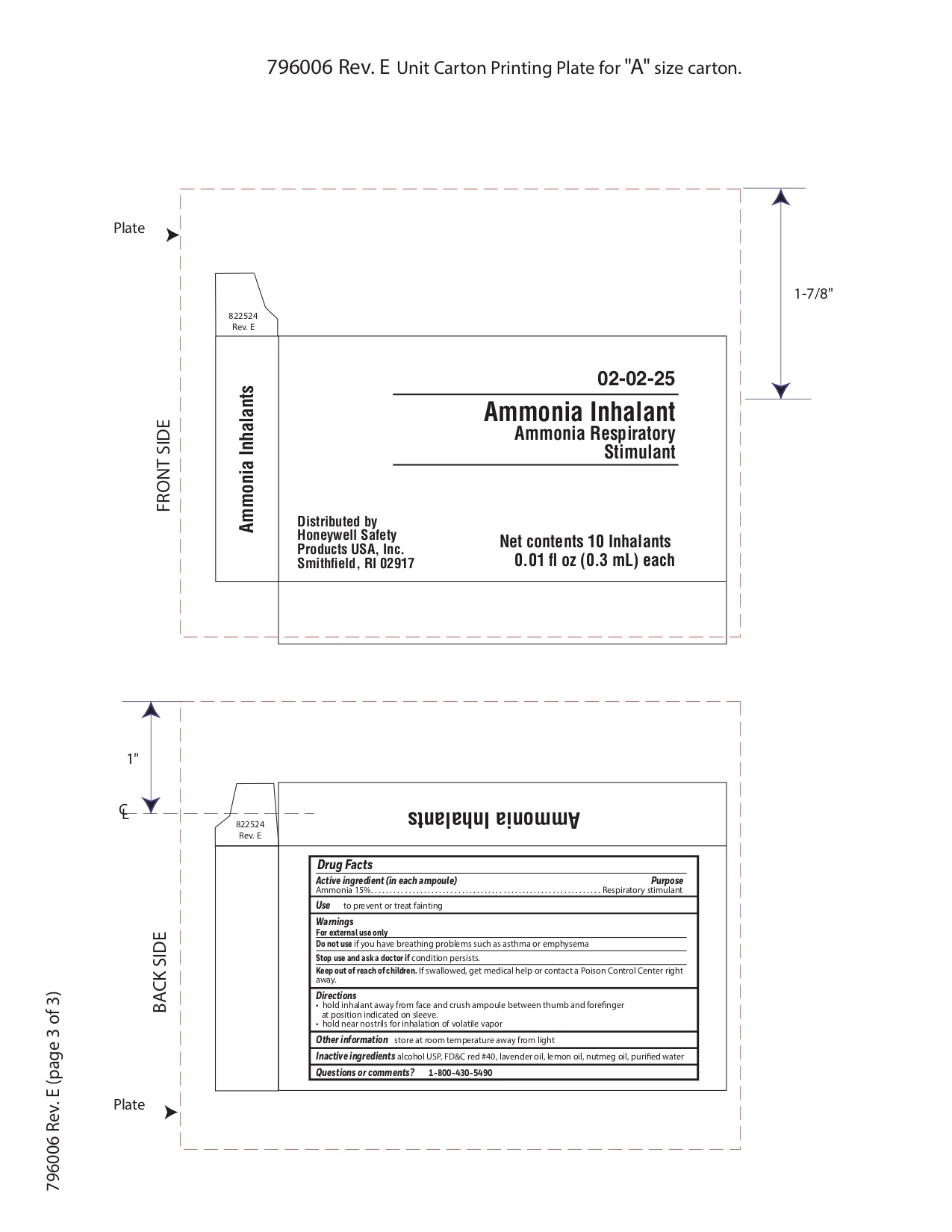
Ammonia
Active ingredient
Ammonia 15%
Ammonia
Purpose
Respiratory stimulant
Ammonia
Uses
- to prevent or treat fainting
Ammonia
Warnings
For external use only
Do not use
- if you have breathing problems such as asthma or emphysema
Stop use and ask a doctor if
Keep out of reach of children
- If swallowed get medical help or contact a Poison Control Center right away.
Ammonia
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Ammonia
Other information
- store at room temperature away from light
Ammonia
Inactive ingredient
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
Ammonia
Questions or Comments?
1-800-430-5490
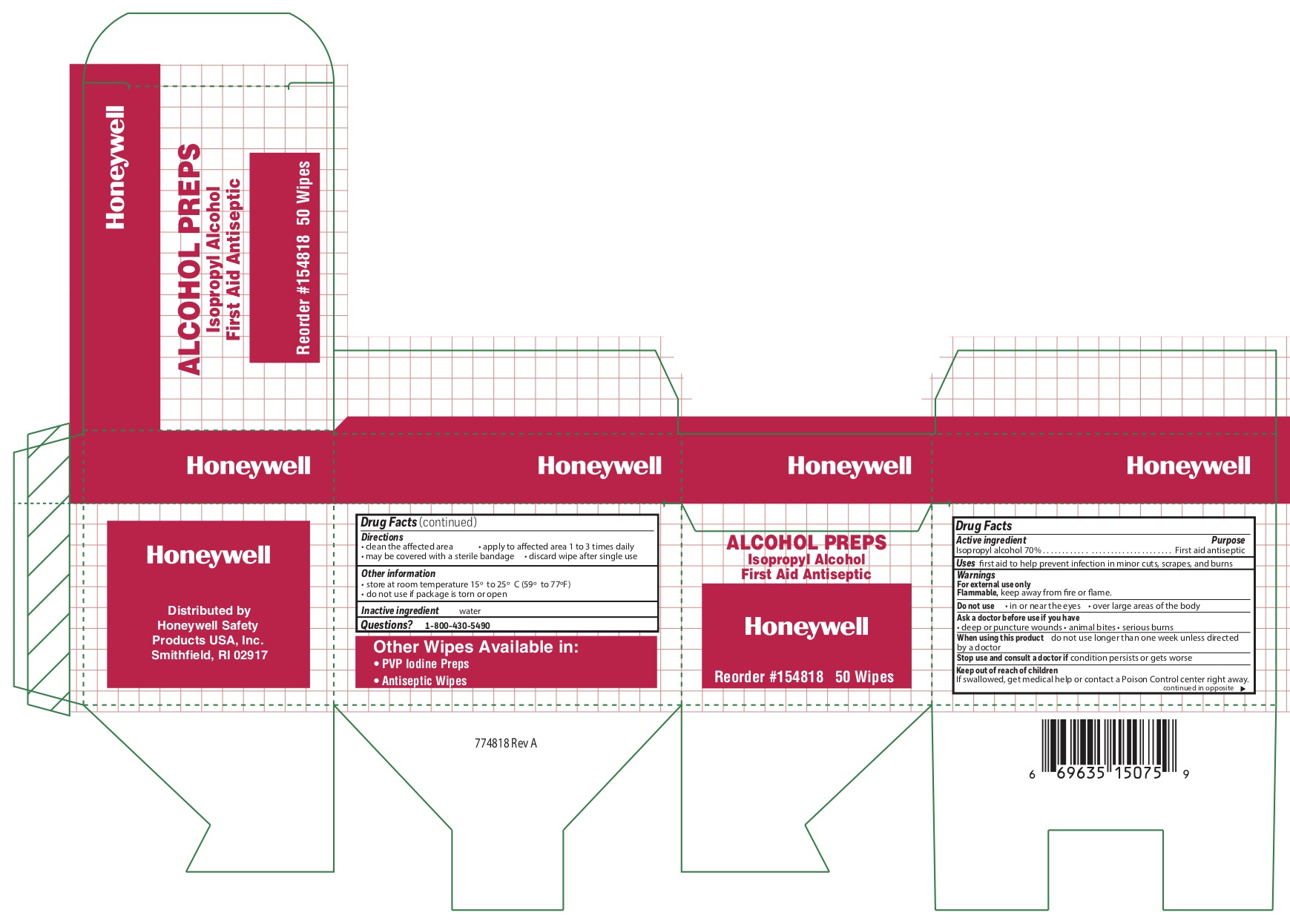
Alcohol Wipes
Active ingredient
Isopropyl alcohol 70%
Alcohol Wipes
Purpose
First aid antiseptic
Alcohol Wipes
Uses
first aid to help prevent infection in minor cuts, scrapes, and burns
Alcohol Wipes
Warnings
For external use only
Flammable, keep away from fire and flame
Do not use
- in or near eyes
- over large areas of the body
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
When using this product
- do not use longer than 1 week unless directed by a doctor
Stop use and consult a doctor if
- condition persists or gets worse
Keep out of the reach of children
If swallowed, get medical help or contact a Poison Control center right away
Alcohol Wipes
Directions
- clean the affected area
- may be covered with a sterile bandage
- apply wipe to affeted are 1 to 3 times daily
- discard wipe after single use
Alcohol Wipes
Other information
- store at room temperature 15
0 to 25
0 C (59
0 to 77
0 F)
- do not use if packet is torn or opened
Alcohol Wipe
Inactive ingredient
water
Alcohol Wipe
Questions
1-800-430-5490
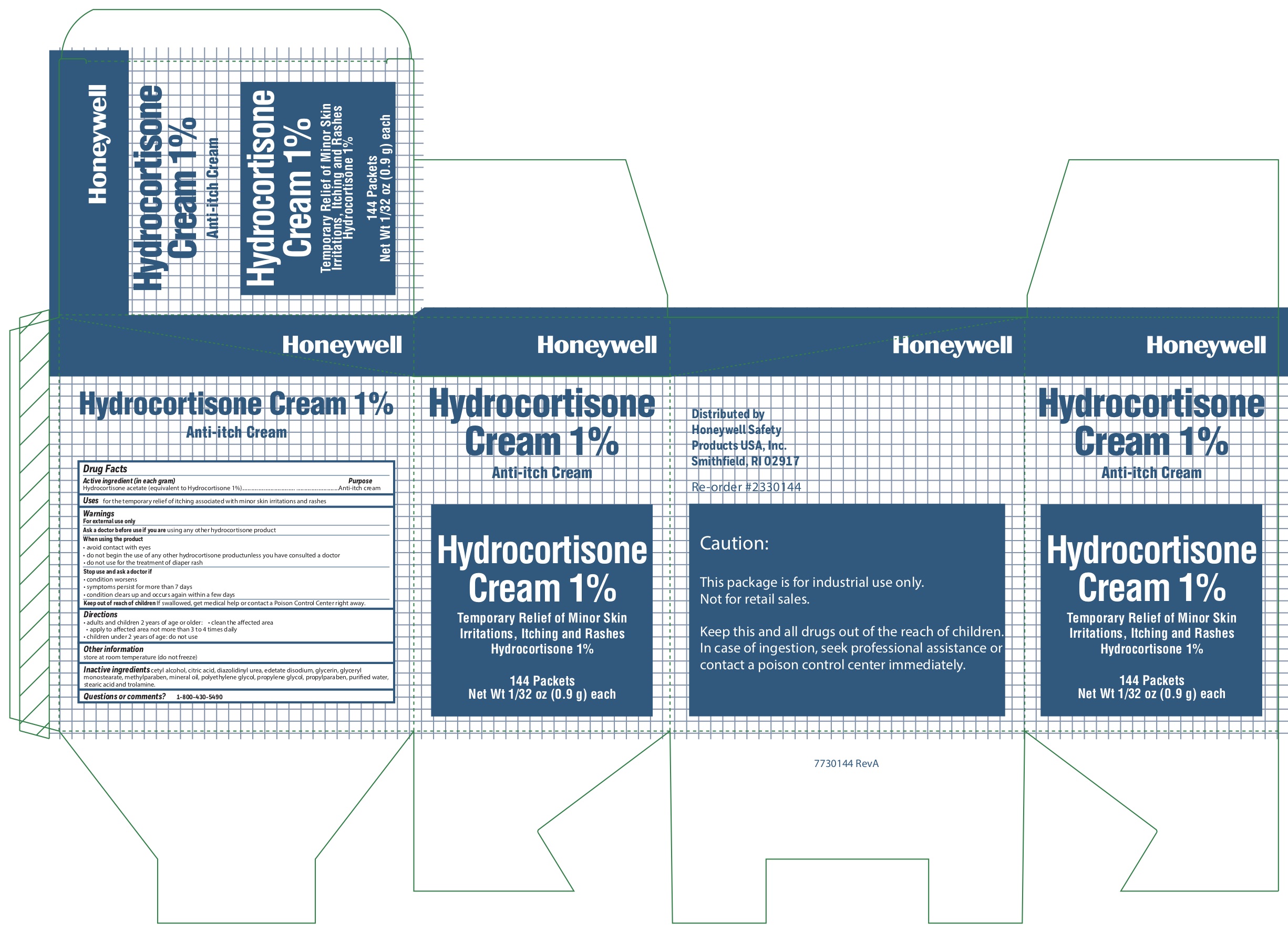
Hydrocortisone
Acitive ingredient (in each gram)
Hydrocortisone acetate (equivalent to Hydrocortisone 1%)
Hydrocortisone
Purpose
Anti-itch cream
Hydrocortisone
Uses
for the temporary relief of itching associated with minor skin irritations and rashes
Hydrocortisone
Warnings
For external use only
Ask a doctor before use if
- you are using any other hydrocortisone product
When using the product
- avoid contact with eyes
- do not begin use of any other hydrocortisone product unless you have consulted a doctor
- do not use for the treatment of diaper rash
Stop use and ask a doctor if
- condition worsens
- condition persists for more than 7 days
- condition clears up and recurs within a few days
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Hydrocortisone
Directions
-
adults and children 2 years and older
- clean the affected area
- apply to the area not more than 3 to 4 times daily
- chil
:dren under 2 years of age: consult a doctor
Hydrocortisone
Other information
- store at room temperature (do not freeze)
Hydrocortisone
Inactive ingredients
cetyl alcohol, citric acid, diazolidinyl urea, edetate disodium, glycerin, glyceryl monostearate, methylparaben, mineral oil, polyethylene glycol, propylene glycol, propylparaben, purified water, stearic acid, trolamine
Hydrocortisone
Questions or Comments?
1-800-430-5490
PVP Wipe
Active ingredient
Povidone-iodine solution USP, 10% (equivalent to 1% titratable iodine)
PVP Wipe
Purpose
First aid antiseptic
PVP Wipe
Uses
- first aid to help prevent the risk of infection in minor cuts, scrapes, and burns
PVP Wipe
Warnings
For external use only
Do not use
- in the eyes
- over large areas of the body
- on individuals who are allergic or sensitive to iodine
Ask a doctor before use if you have
- deep or puncture wounds,
- animal bites
- serious burns
When using this product
- do not use longer than one wek unless directed by a doctor
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away
Stop use and ask a doctor if
- conditions persists or gets worse
- irritation and redness develops
PVP Wipe
Directions
Reverse cardboard sleeve, then crush at dot between thumb and forefinger. Allow solution to saturate tip and apply solution to injury.
- clean affected area
- apply to affected area 1 to 3 times daily
- may be covered with a sterile bandage
- discard swab after single use
PVP
Other information
- store at room temperature away from light
- keep from freezing or excessive heat
- do not use if package is torn or open
PVP
Inactive ingredients
citric acid, disodium phosphate,nonoxynol-9, sodium hydroxide, water
PVP
Questions and Comments?
1-800-430-5490
Burn Relief
Active ingredient
Lidocaine HCL 2%
Burn Relief Spray
Purpose
External analgesic
Burn Spray
Uses
temporarily relieves pain due to minor burns
Burn Relief Spray
Warnings
For external use only
Do not use
- over large areas of the body, particularly over raw surfaces or blistered areas
When using this product
- avoid contact with the eyes
Stop use and ask a doctor if
- condition worsens
- symptoms persist for more than 7 days
- condition clears up and occurs again within a few days
Keep out of the reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Burn Relief
Directions
adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
children under 2 years of age: ask a doctor
Burn Relief Spray
Other information
- store at room temperature
Burn Reelief Spray
Inactive ingredients
diazolidinyl urea, edetate disodium, glycerin, hypromellose, methylparaben, octoxynol 9, propylene glycol, propylparaben, purified water, tea tree oil, trolamine
Burn Relief Spray
Questions or Comments?
1-800-430-5490
BZK
Active ingredient
Benzalkonium chloride 0.13% w/v
BZK
Purpose
First aid antiseptic
BZK
Uses
Antiseptic cleansing of face, hands, and body without soap and water
BZK
Warnings
For external use only
Stop use and ask a doctor if
- if irritation, redness or other symptoms develop
- the condition persists or gets worse
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
BZK
Directions
tear open packet and use as a washcloth
BZK
Other iformation
- store at room temperature 15
0 to 30
0 C (59
0 - 86
0 F)
- do not reuse towelette
BZK
Inactive ingredient
water
BZK
Questions
1-800-430-5490
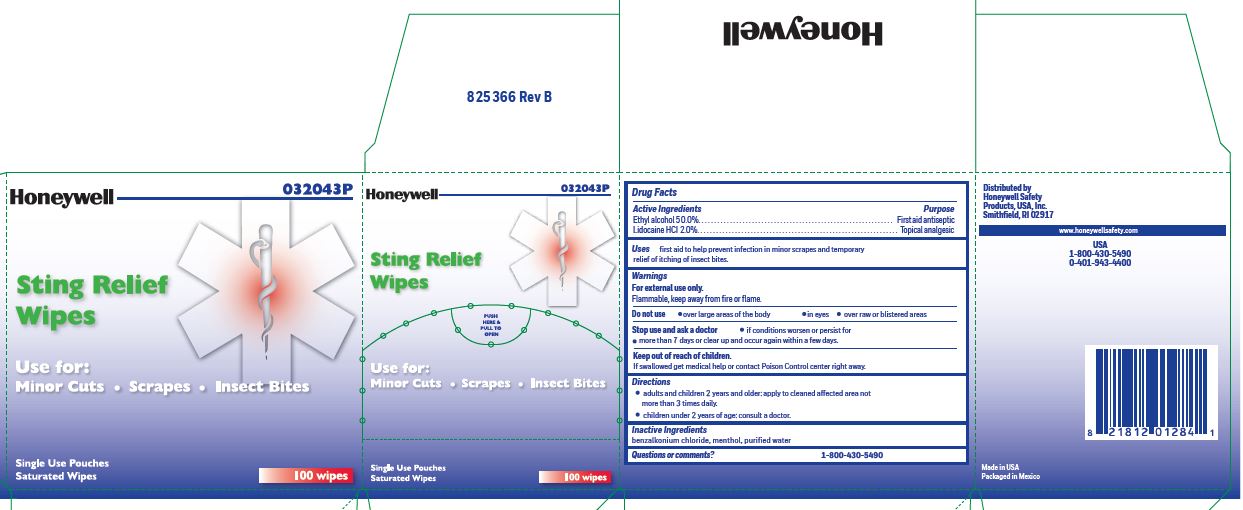
Sting Relief
Active ingredient (in each wipe)
Ethyl alcohol 50.0%
Lidocaine HCl 2.0%
Sting Relief
Purpose
Antiseptic
Topical pain relief
Sting Relief
Uses
prevent infection in minor scrapes, and temporary relief of itching of insect bites
Sting Relief
Warnings
For external use only
Flammable, keep away from open fire or flame
Do not use
over large areas of the body
in eyes
over raw or blistered areas
Stop use and ask a doctor
- if conditions worsen or persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Sting Relief
Directions
- adults and children 2 years and older: Apply to cleaned affected area not more than 3 times daily.
- children under 2 years of age: consult a doctor.
Sting Relief
Inactive ingredients
benzalkonium chloride, menthol, and purified water
Sting Relief
Questions or Comments?
1-800-430-5490
Tripe
Active ingredients (each gram contains)
Bacitracin zinc 400 units
Neomycin sulfate (5 mg equivalent to 3.5 mg Neomycin base)
Polymyxin B sulfate 5000 units
Triple
Purpose
First aid antibiotic
First aid antibiotic
First aid antibiotic
Triple
Uses
- first aid to help prevent infection in
- minor cuts
- scrapes
- burns
Triple
Warnings
For external use only
Allergy alert do not use if you are allergic to any of the ingredients
Do not use
- in the eyes
- over large areas of the body
Ask a doctor before use if you have
- a deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- the condition persists or gets worse
- a rash or other allergic reaction develops
- you need to use longer than 1 week
Keep out of the reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Triple
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Triple
Other information
- store at 15
0 to 25
0 C (59
0 to 77
0 F)
- tamper evident sealed packets
- do not use if packet is torn or opened
Triple
Inactive ingredient
petrolatum
Triple
Questions?
1-800-430-5490
4365
SF00004081 Kit Contents
1 SWIFT 1" X 3" PLAS 100/BOX
1 FINGERTIP "T" WOVEN 40/BOX
1 1X3 WOVEN SING 50/BOX
1 SWIFT KNUCKLE 40/BX
1 AMMONIA INHALANTS 10 PER
1 INSTANT COLD PACK 4" X 6"
1 ALCOHOL PREP PADS 10P
1 HYDROCORTISON,1.O%,1/32 OZ,10P
1 PVP IODINE WIPES 10 PER
1 STING RELIEF WIPES 10 PER BOX
1 BIOHAZARD BAGS
1 ELASTIC TAPE 1" X 5YD
1 O/H TAPE ADHESIVE TRI-CUT
1 O/H PUMP BURN RELIEF 2 OZ ID G
1 FIRST AID GUIDE ASHI
4 GAUZE CLEAN-WRAP BDGE N/S 2"
1 BLOODSTOPPER
1 GZE PADS STERILE 4"X 4" 10'S
1 GZE PADS STERILE 2"X 2" 25'S
1 CO-FLEX BANDAGE 2"X 5YDS TAN
1 CPR FILTERSHIELD 77-100
1 COTTON TIPS 100 PER VIAL
1 ANTISEPTIC WIPES BZK CHL 20'S
1 ASPIRIN IND PK 5 GR 2/ENV 250
1 TRIPLE BIOTIC .5 GRAM PKT 20
1 POISON OAK/IVY CLEANSER 4 OZ
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
1 F A KIT EMPTY BLANK 140
1 POCKET INSERT RED #140 KIT 2R
1 BANDAGE COMP 2" W/TELFA PAD 4
1 TONGUE BLADES SR WRAPPED 6'S
2 PR LRG NITRILE GLVES ZIP BAG
6 WATER-JEL BURN DRESSING 2 X 6
6 WATER-JEL BURN DRESSING 2 X 2
2 TRI BNDG NON WOVEN 40"X40"X56"
10 NON ADHERENT PAD 2" X 3"

Aspirin
Principal Display Panel

Ammonia
Principal Display Panel
Alcohol Wipe
Principal Display Panel
Hydrocortisone
Principal Display Panel
PVP
Principal Display Panel

Burn Relief Spray
Principal Display Panel

BZK
Principal Display Panel

Sting Relief
Principal Display Panel
Triple
Principal Display Panel
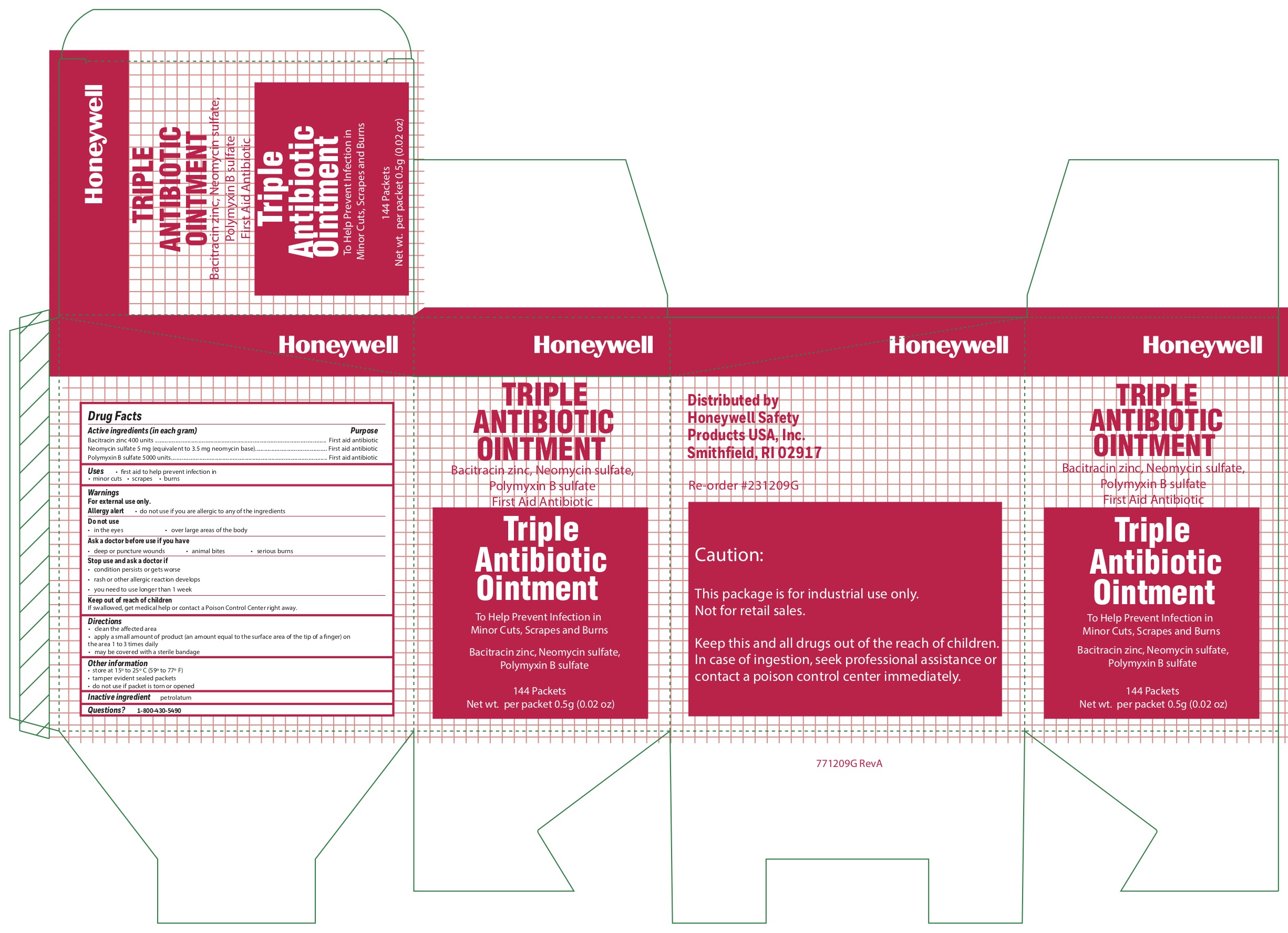
4365 Kit Label
SF00004081











