Eyewash
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyewash
Warnings
For external use only Obtain immediate medical treatment for all open wounds in or near eyes. To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Eyewash
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
Burn Relief Water Soluble
Active ingredients
Benzethonium chloride 0.2% w/w
Benzocaine 10% w/w
Menthol 0.33% w/w
Burn Relief Water Soluble
Uses
for the temporary relief of pain and itching and helps protect against infection in:
- minor cuts and scrapes
- burns
- sunburn
- insect bites
- minor skin irritations
Burn Relief Water Soluble
Warnings
For external use only
Flammable keep away from fire or flame
- contents under pressure
- do not puncture or incinerate container
- do not expose to temperatures above 120 0 F
Do not use
- in or near the eyes or other mucous membranes
- in case of serious burns
- in case of deep or puncture wounds
- for prolonged period of time
- on large portion of the body
Burn Relief Water Soluble
Directions
- clean the affected area
- shake can well before using
- hold 4 - 6 inches from surface and spray area until wet
- may be covered with a sterile bandage, if bandaged let dry first
- for adult institutional use only
- not intended for use on children
Burn Relief Water Soluble
Other information
- avoid inhaling
- use only as directed
- intentional misuse by deliberately concentrating or inhaling the contents may be harmful or fatal
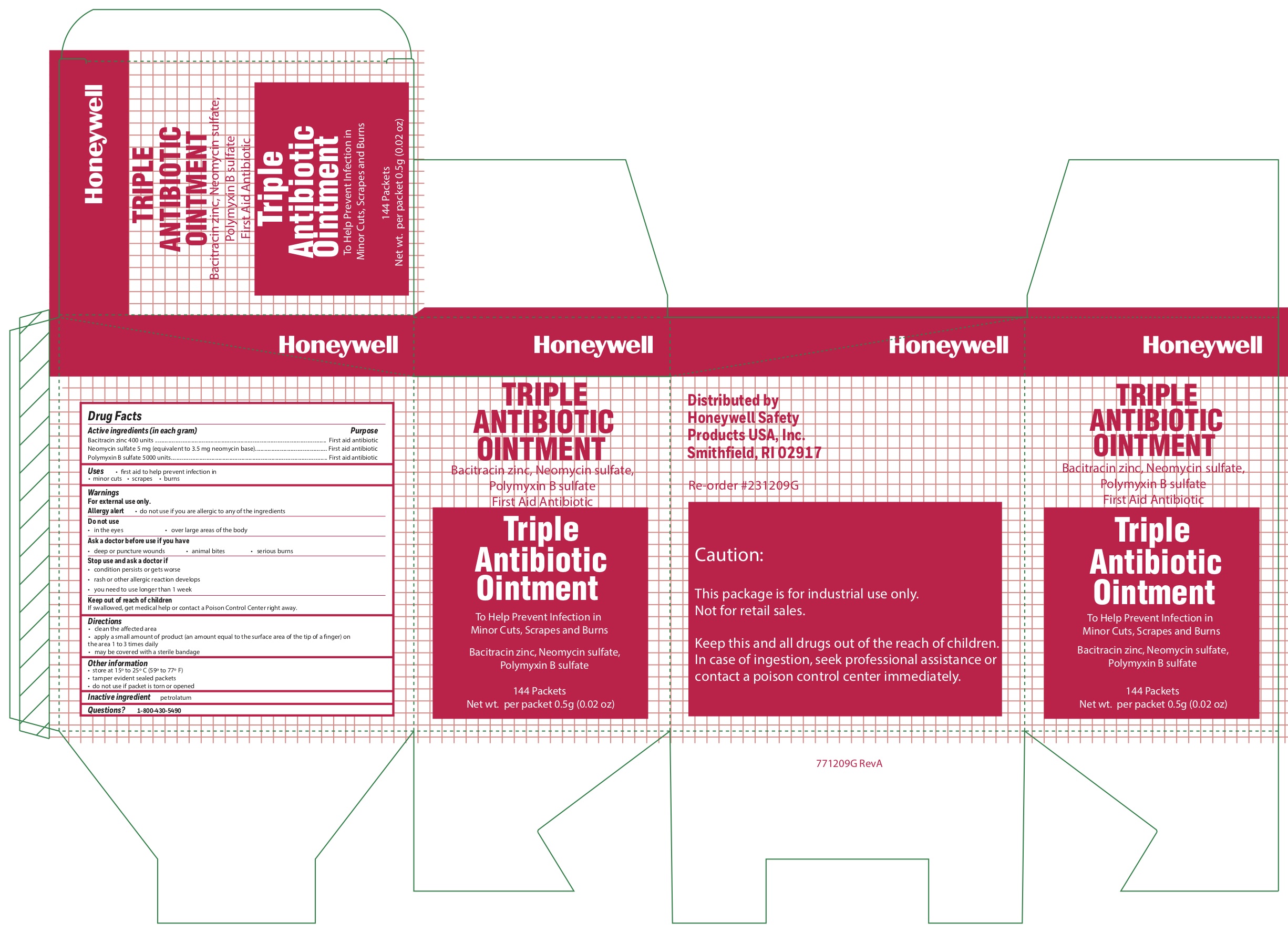
Triple
Active ingredients
Bacitracin zinc 400 units
Neomycin sulfate (5 mg equivalent to 3.5 mg Neomycin base)
Polymyxin B sulfate 5000 units
Triple
Warnings
For external use only
Allergy alert do not use if you are allergic to any of the ingredients
Triple
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Triple
Other information
- store at 15 0 to 25 0 C (59 0 to 77 0 F)
- tamper evident sealed packets
- do not use if packet is torn or opened
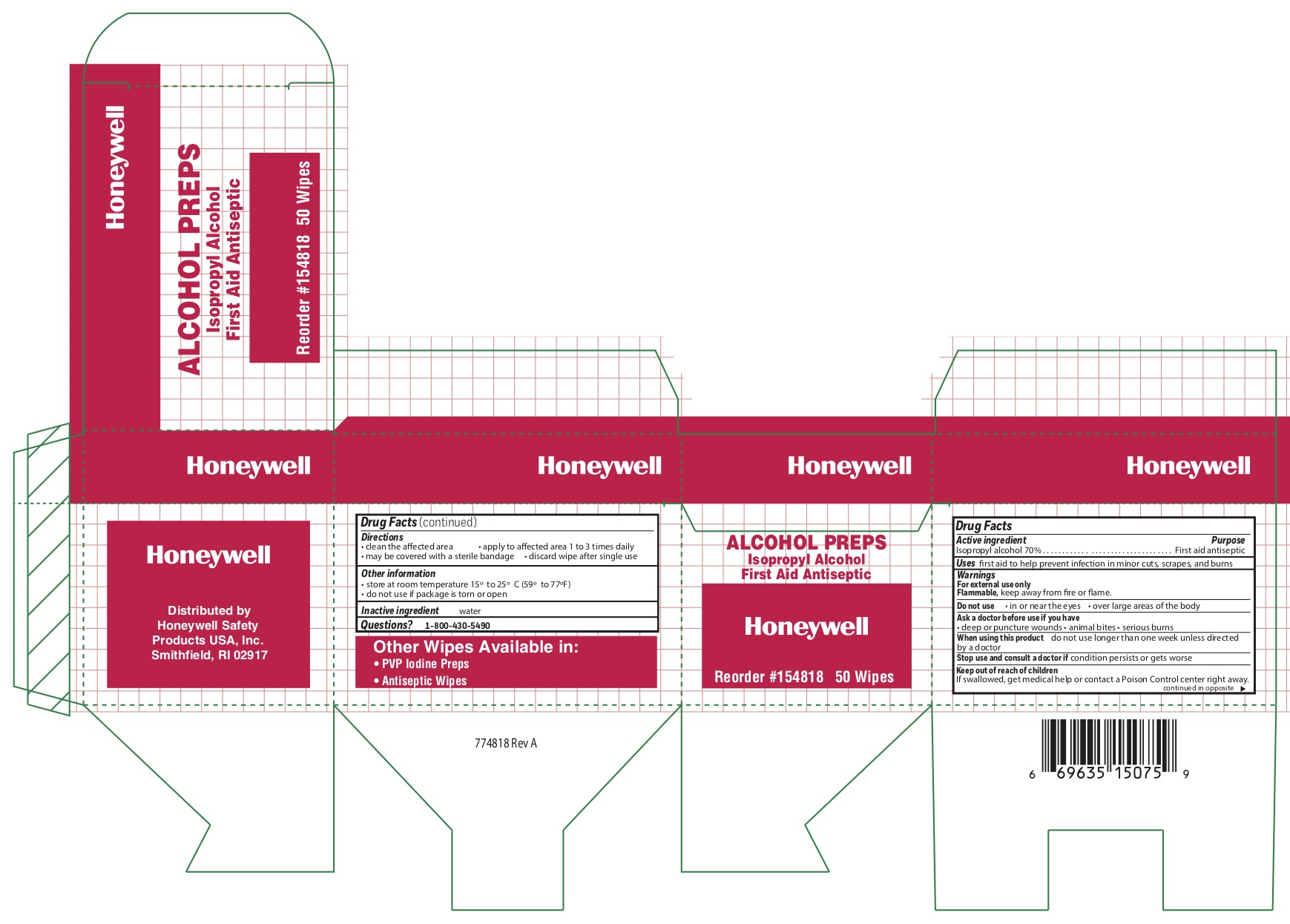
Alcohol
Directions
- clean the affected area
- apply wipe to affected area 1 to 3 times daily
- may be covered with a sterile bandage
- discard wipe after single use
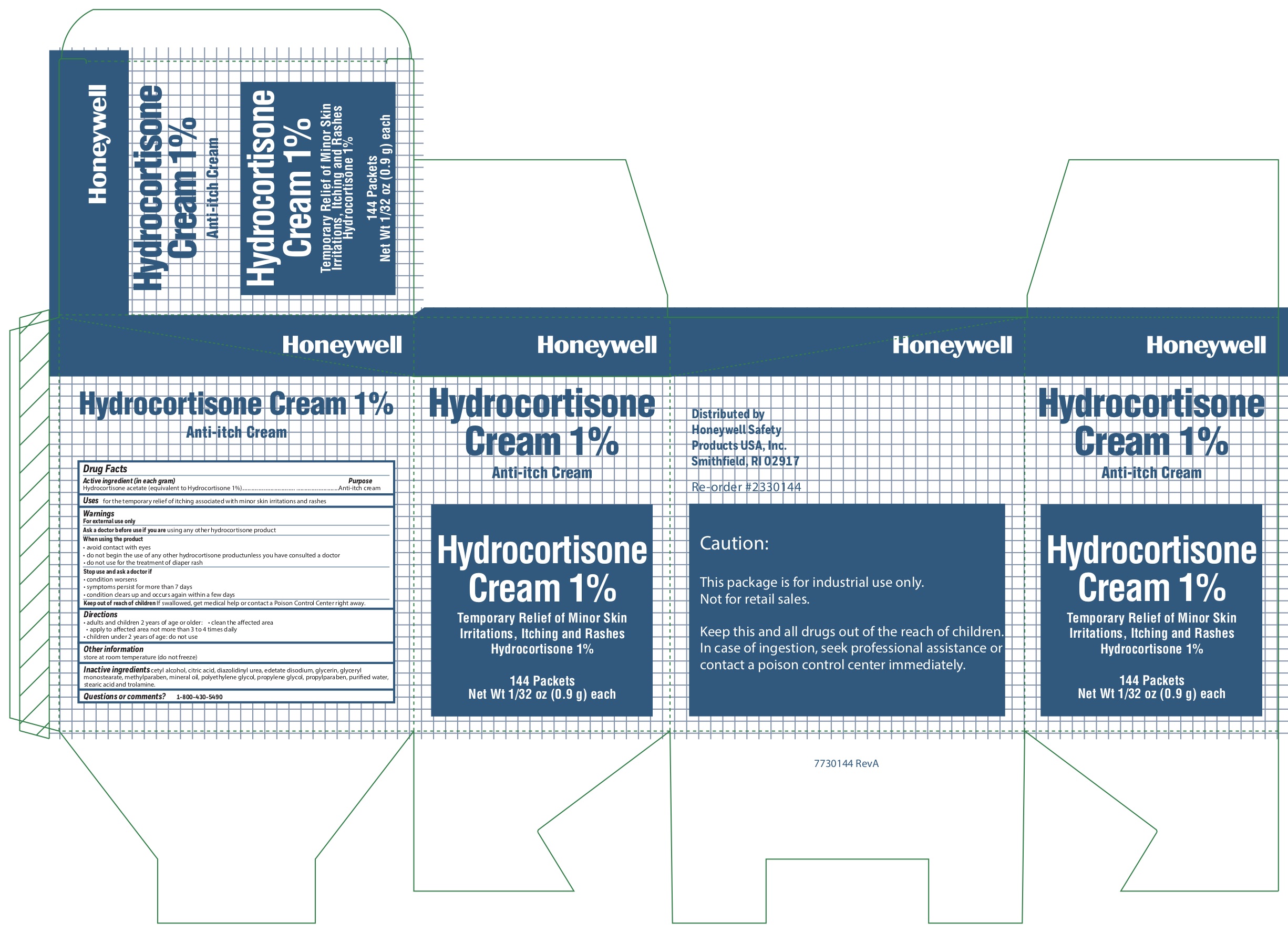
Hydrocortisone
Active ingredient (in each gram)
Hydrocortisone acetate (equivalent to Hydrocortisone 1%)
Hydrocortisone
Uses
- for the temporary relief of itching associated with minor skin irritations and rashes
Hydrocortisone
Warnings
For external use only
When using the product
- avoid contact with eyes
- do not begin use of any other hydrocortisone product unless you have consulted a doctor
- do not use for the treatment of diaper rash
Hydrocortisone
Directions
- adults and children 2 years and older:
- clean the affected area
- apply to the area not more than 3 to 4 times daily
- children under 2 years of age: consult a doctor
Hydrocortisone
Inactive ingredients
cetyl alcohol, citric acid, diazolidinyl urea, edetate disodium, glycerin, glyceryl monostearate, methylparaben, mineral oil, polyethylene glycol, propylene glycol, propylparaben, purified water, stearic acid, trolamine
Foille
Uses
- For the temporary relief of pain associated with burns, sunburn, minor cuts, scrapes, insect bites, and minor skin irritations.
- First aid to help prevent infection in minor cuts, scrapes and burns.
Foille
Warnings
For external use only
Foille
Directions
- clean the affected area.
- adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily.
- children under 2 years of age: consult a physician.
Foille
Inactive ingredients
beeswax, benzyl alcohol, calcium disodium EDTA, calcium hydroxide, ceresin, eugenol, hydrogenated vegetable oil, maleic anhydride, mono- and di-glycerides, PEG-32, purified water, sodium borate, sodium lauryl sulfate, zea mays (corn) oil.
Burn Jel
Warnings
For external use oonly
Burn Jel
Directions
- adults and children 2 years of age and older; apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
- you may report a serious reaction to this product to 800-430-5490
Burn Jel
Inactive ingredients
carbopol 940, carbopol 1342, diazolidinyl urea, glycerin, melaleuca alternifolia (tea tree) leaf oil, methylparaben, octoxynol-9, propylene glycol, propylparaben, trolamine, water
4357
6832649 KIT CONTENTS
1 BLUE DETEC FNGERTP 8 WVN 25/B
1 BLUE DETEC KNUCKLE WVN 40/BX
1 BLUE DETEC 1X3 WVN 100/BX
1 INSTANT COLD PACK 4" X 6"
5 BURN JEL 1/8 OZ, 6 PER
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 FIRST AID GUIDE ASHI
1 GAUZE CLEAN-WRAP BDGE N/S 2"
1 GZE PADS STERILE 2"X 2" 25'S
1 GZE PADS STERILE 3"X 3" 25'S
1 CO-FLEX BANDAGE 2"X 5YDS TAN
1 ALCOHOL WIPES 50'S
1 TRIPLE BIOTIC .5 GRAM PKT 20
1 HYDROCORTISONE 1% .9 GRM 20'S
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 SCISSOR BDGE 4" RED PLS HDL
1 SPLINTER OUT 10 PIECES/PK
1 KIT TWEEZER 3 1/2" SLANTED
1 F A KIT EMPTY BLANK 140
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
2 PR LRG NITRILE GLVES ZIP BAG
5 WATER-JEL BURN DRESSING 4 X 4
3 FOILLE BURN .5OZ 2'S