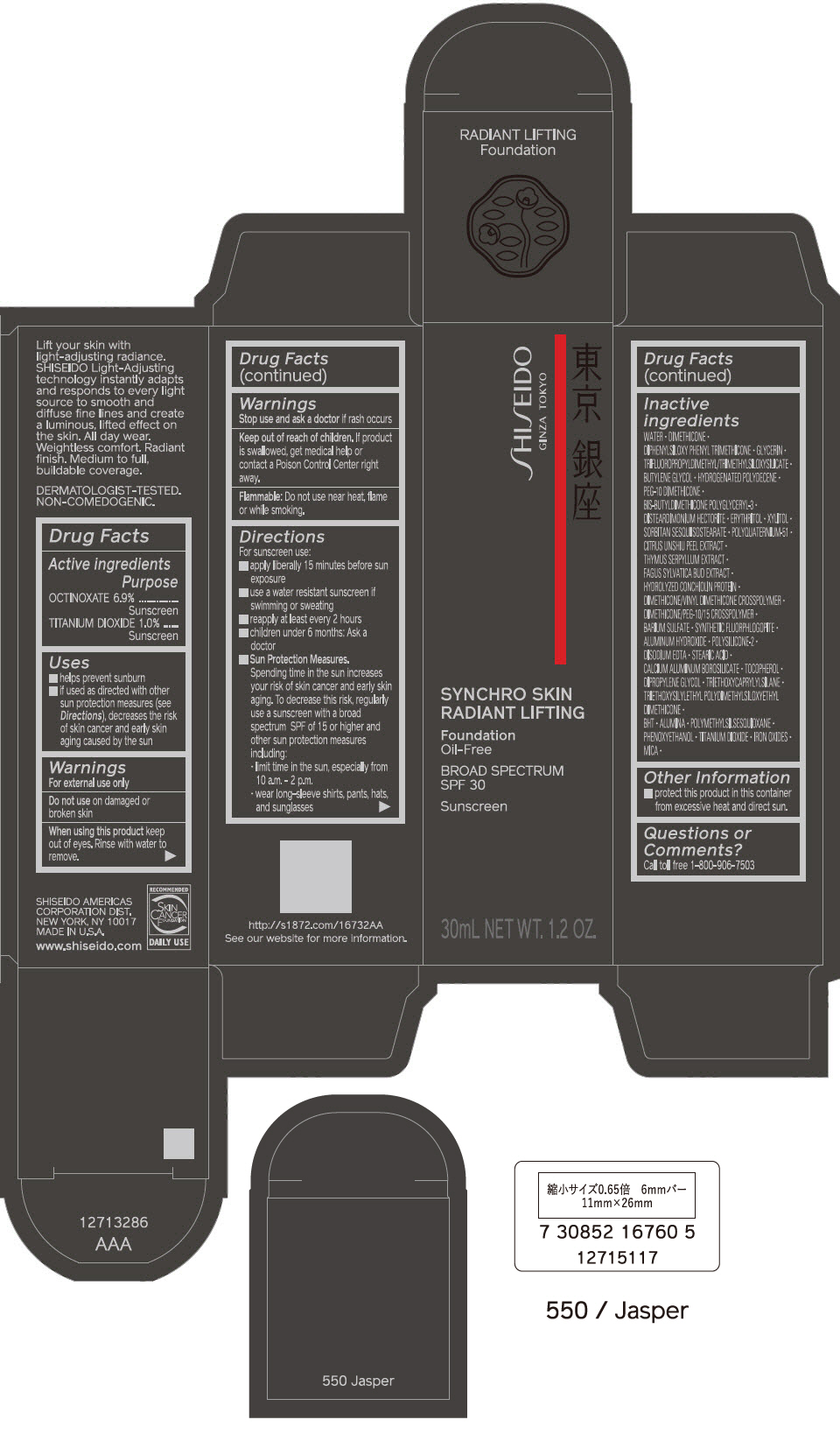
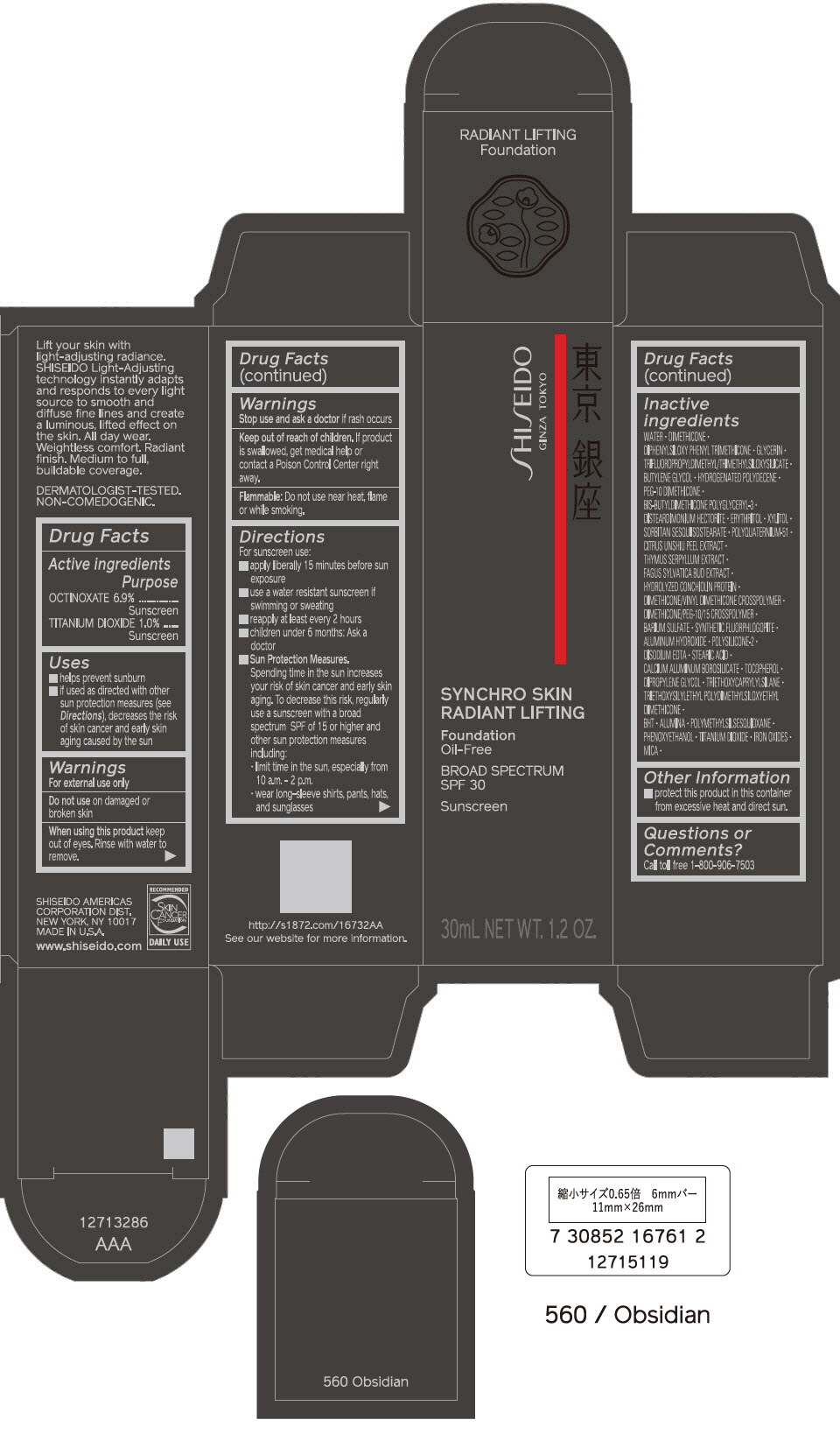
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Warnings
For external use only
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- children under 6 months: Ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF of 15 or higher and other un protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeve shirts, pants, hats, and sunglasses
Inactive Ingredients
WATER▪DIMETHICONE▪DIPHENYLSILOXY PHENYL TRIMETHICONE▪GLYCERIN▪TRIFLUOROPROPYLDIMETHYL/TRIMETHYLSILOXYSILICATE▪BUTYLENE GLYCOL▪HYDROGENATED POLYDECENE▪PEG-10 DIMETHICONE▪BIS-BUTYLDIMETHICONE POLYGLYCERYL-3▪DISTEARDIMONIUM HECTORITE▪ERYTHRITOL▪XYLITOL▪SORBITAN SESQUIISOSTEARATE▪POLYQUATERNIUM-51▪CITRUS UNSHIU PEEL EXTRACT▪THYMUS SERPYLLUM EXTRACT▪FAGUS SYLVATICA BUD EXTRACT▪HYDROLYZED CONCHIOLIN PROTEIN▪DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER▪DIMETHICONE/PEG-10/15 CROSSPOLYMER▪BARIUM SULFATE▪SYNTHETIC FLUORPHLOGOPITE▪ALUMINUM HYDROXIDE▪POLYSILICONE-2▪DISODIUM EDTA▪STEARIC ACID▪CALCIUM ALUMINUM BOROSILICATE▪TOCOPHEROL▪DIPROPYLENE GLYCOL▪TRIETHOXYCAPRYLYLSILANE▪TRIETHOXYSILYLETHYL POLYDIMETHYLSILOXYETHYL DIMETHICONE▪BHT▪ALUMINA▪POLYMETHYLSILSESQUIOXANE▪PHENOXYETHANOL▪TITANIUM DIOXIDE▪IRON OXIDES▪MICA▪
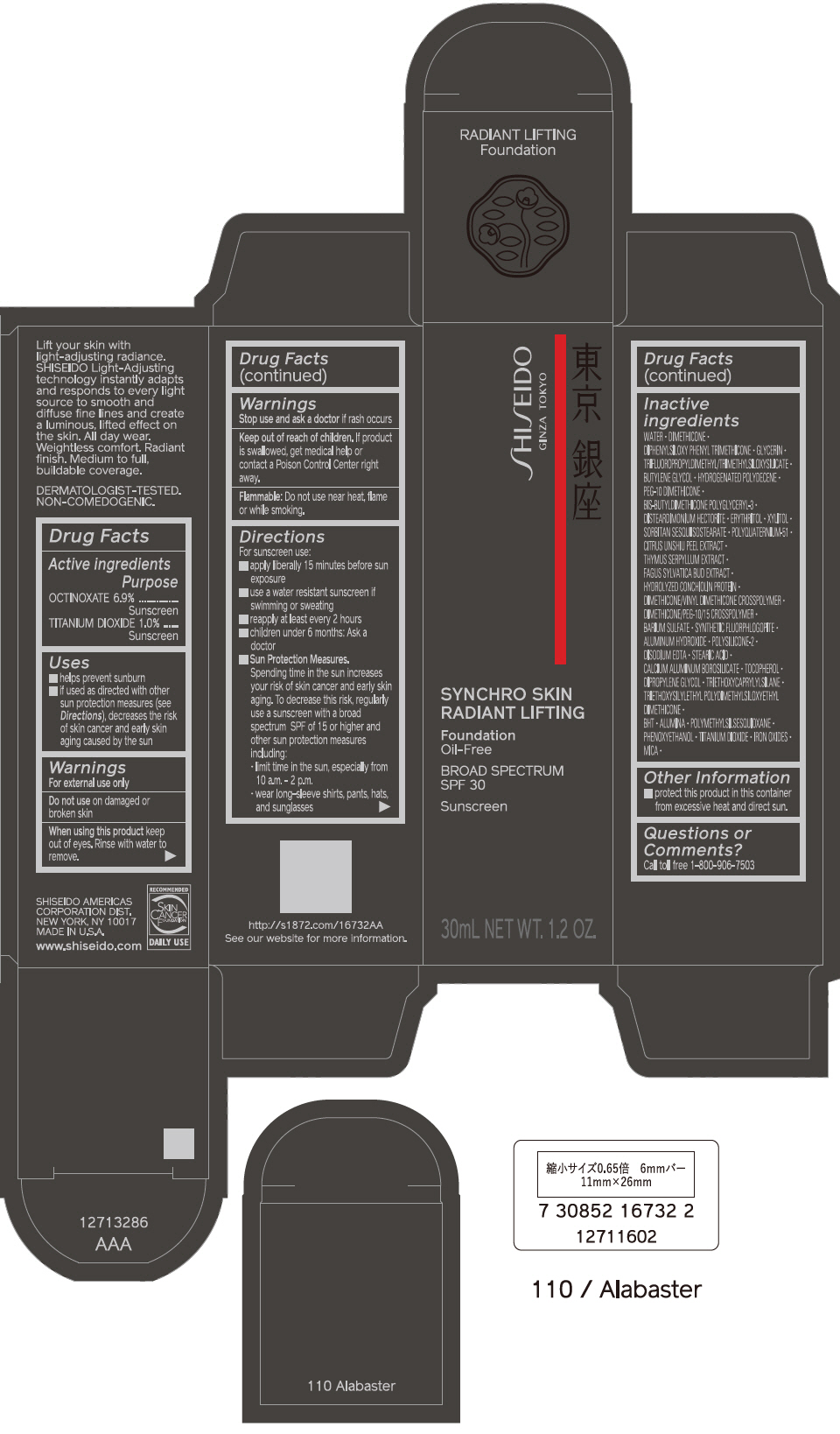
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 110 Alabaster
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 120 Ivory
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
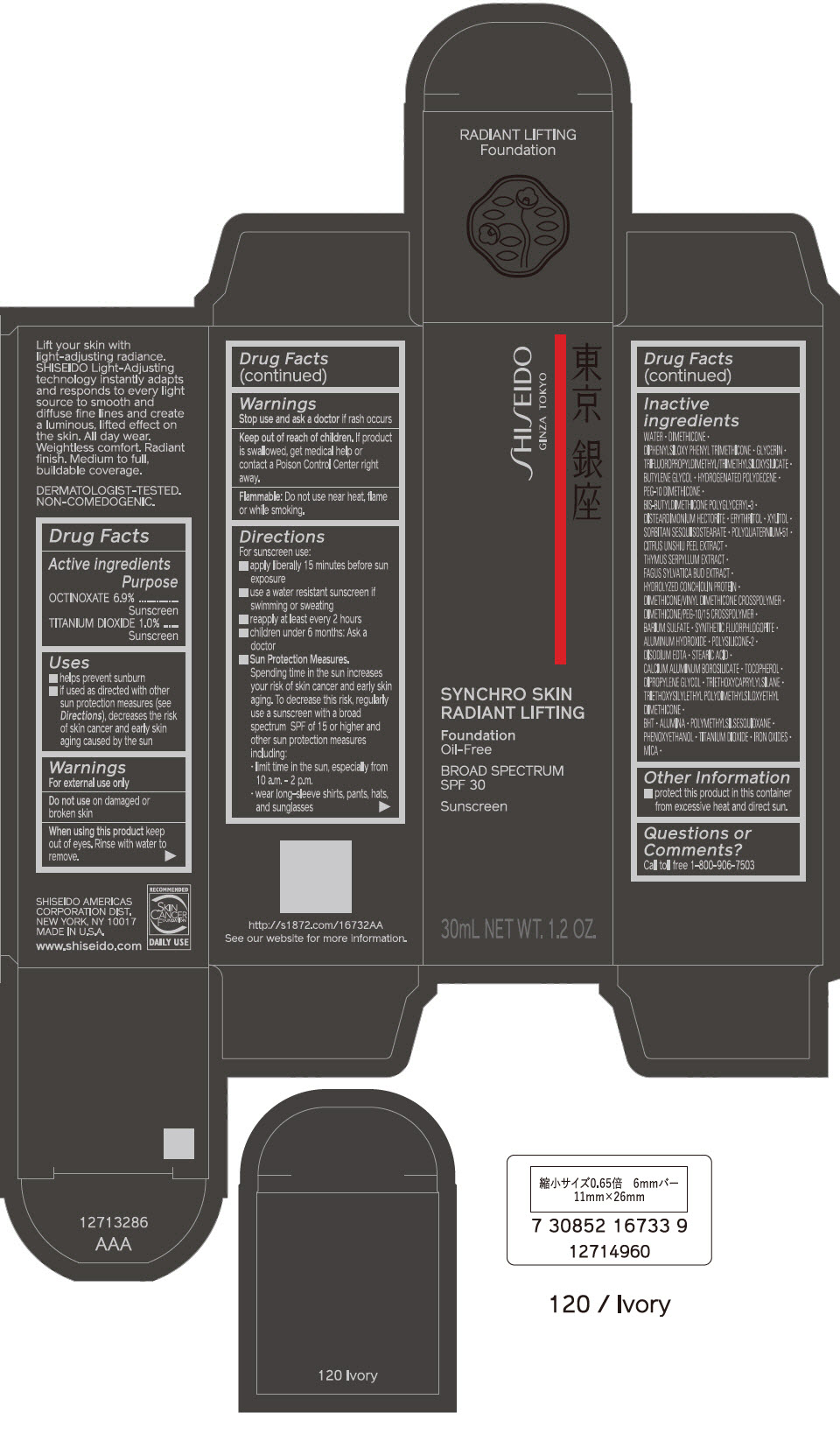
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 130 Opal
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
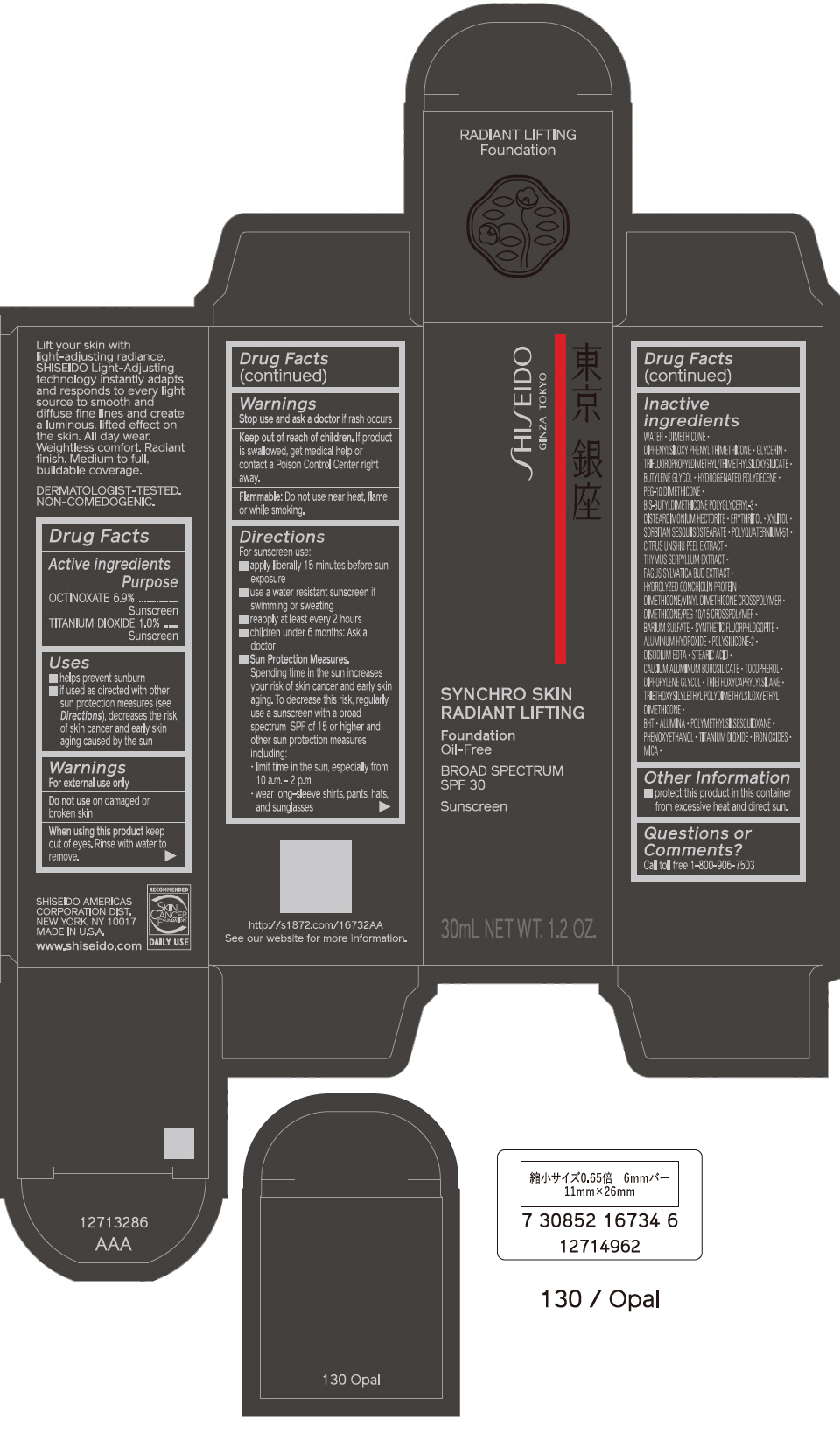
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 140 Porcelain
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
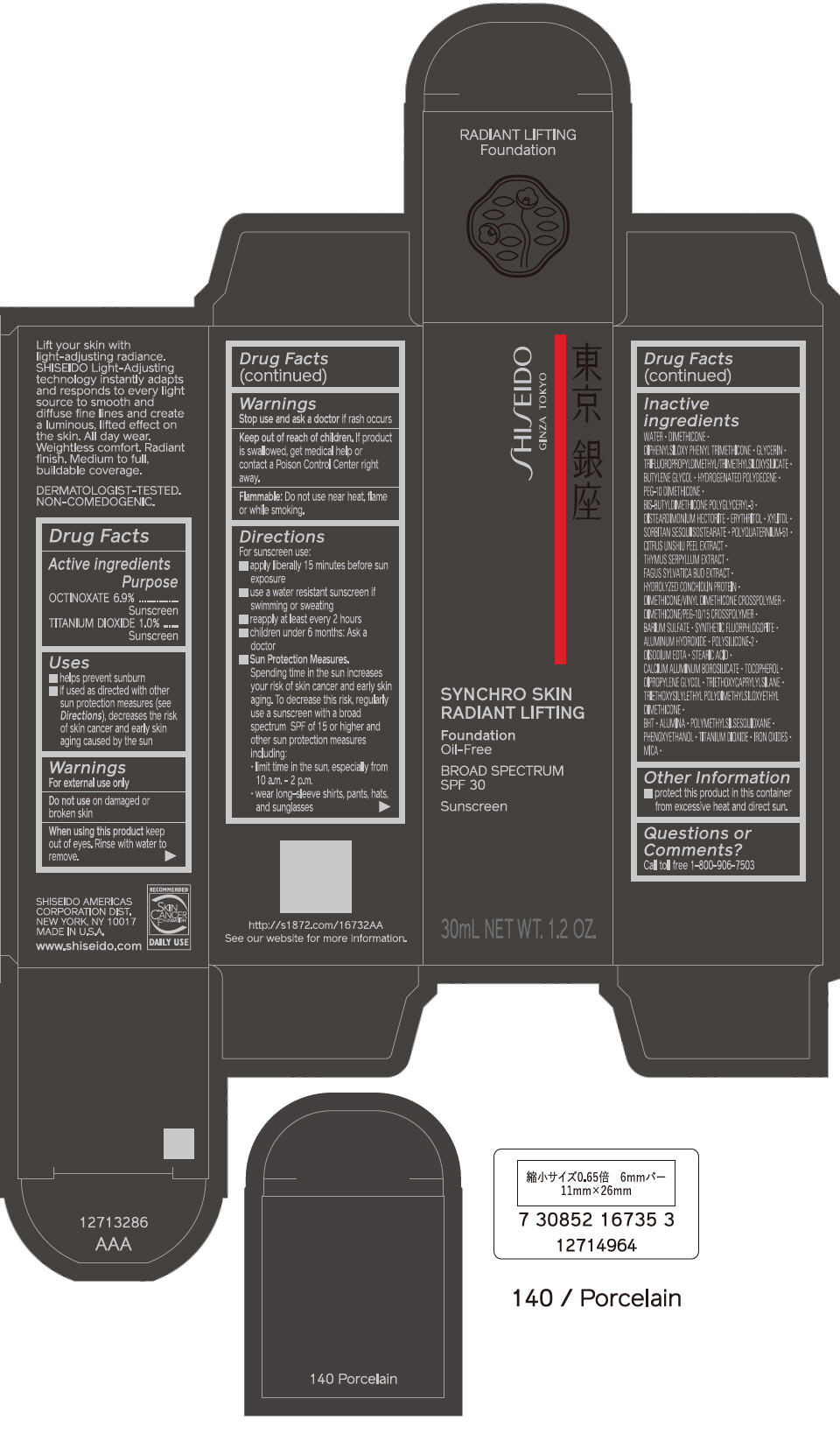
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 150 Lace
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
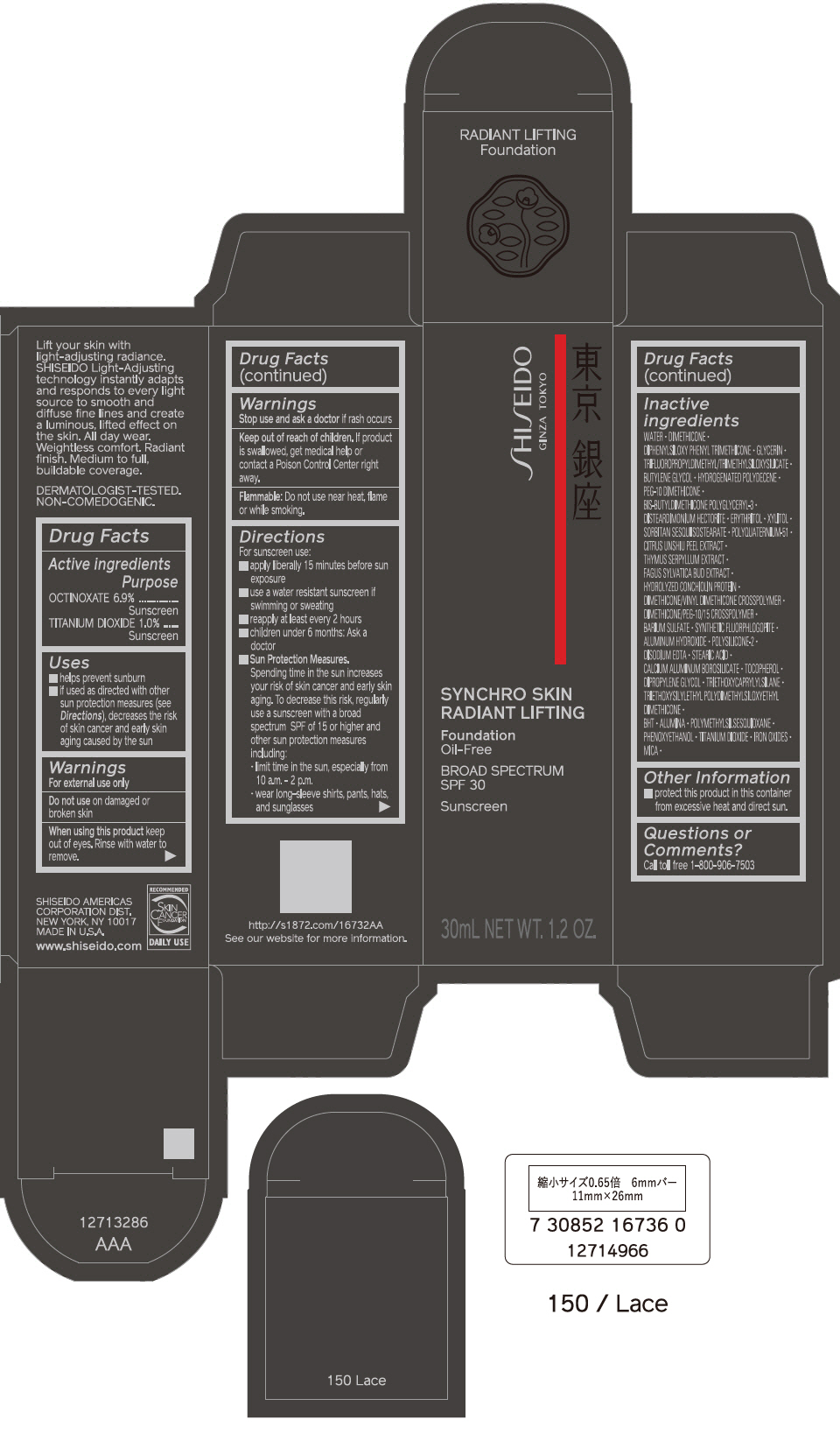
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 160 Shell
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
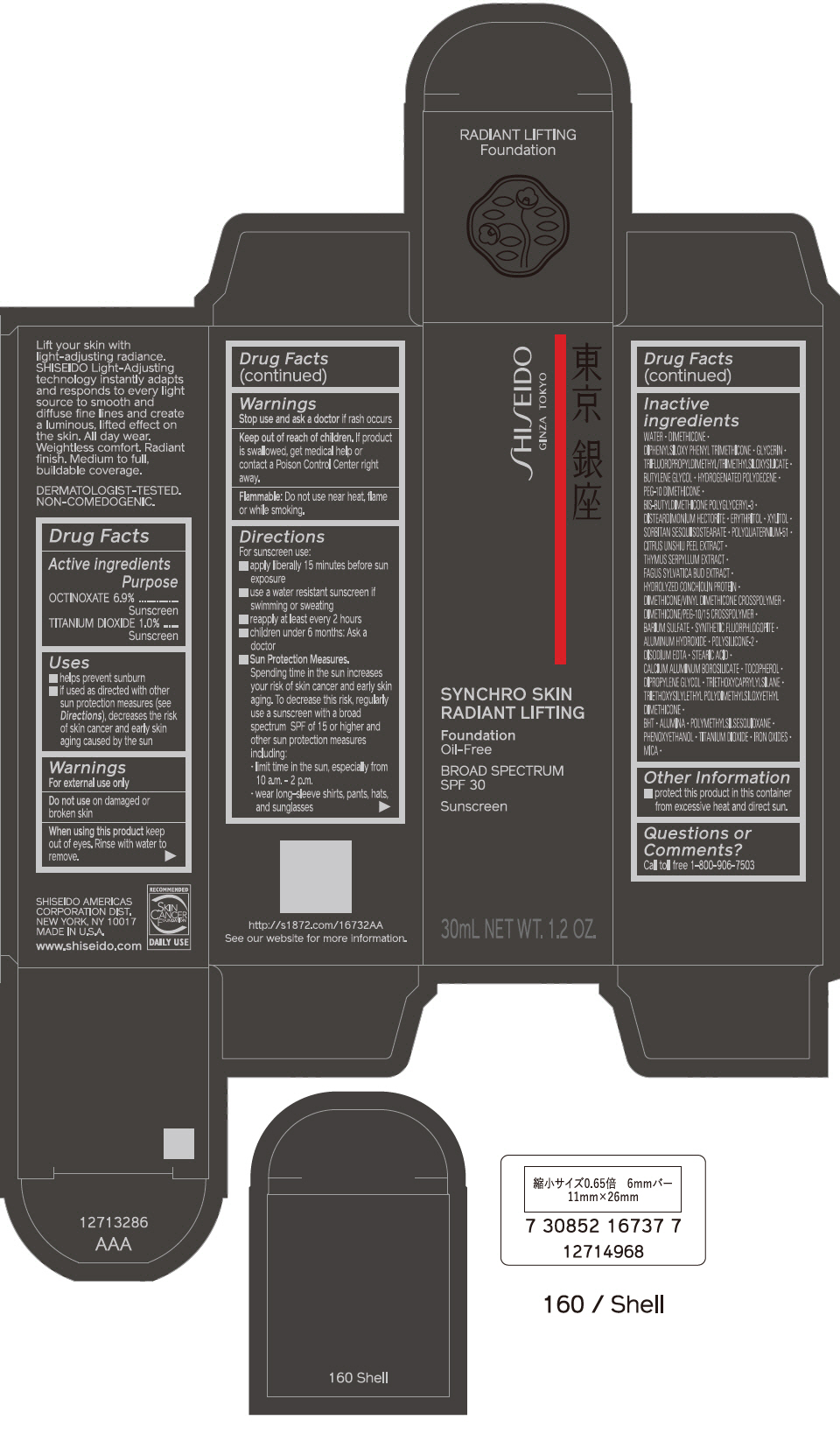
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 210 Birch
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
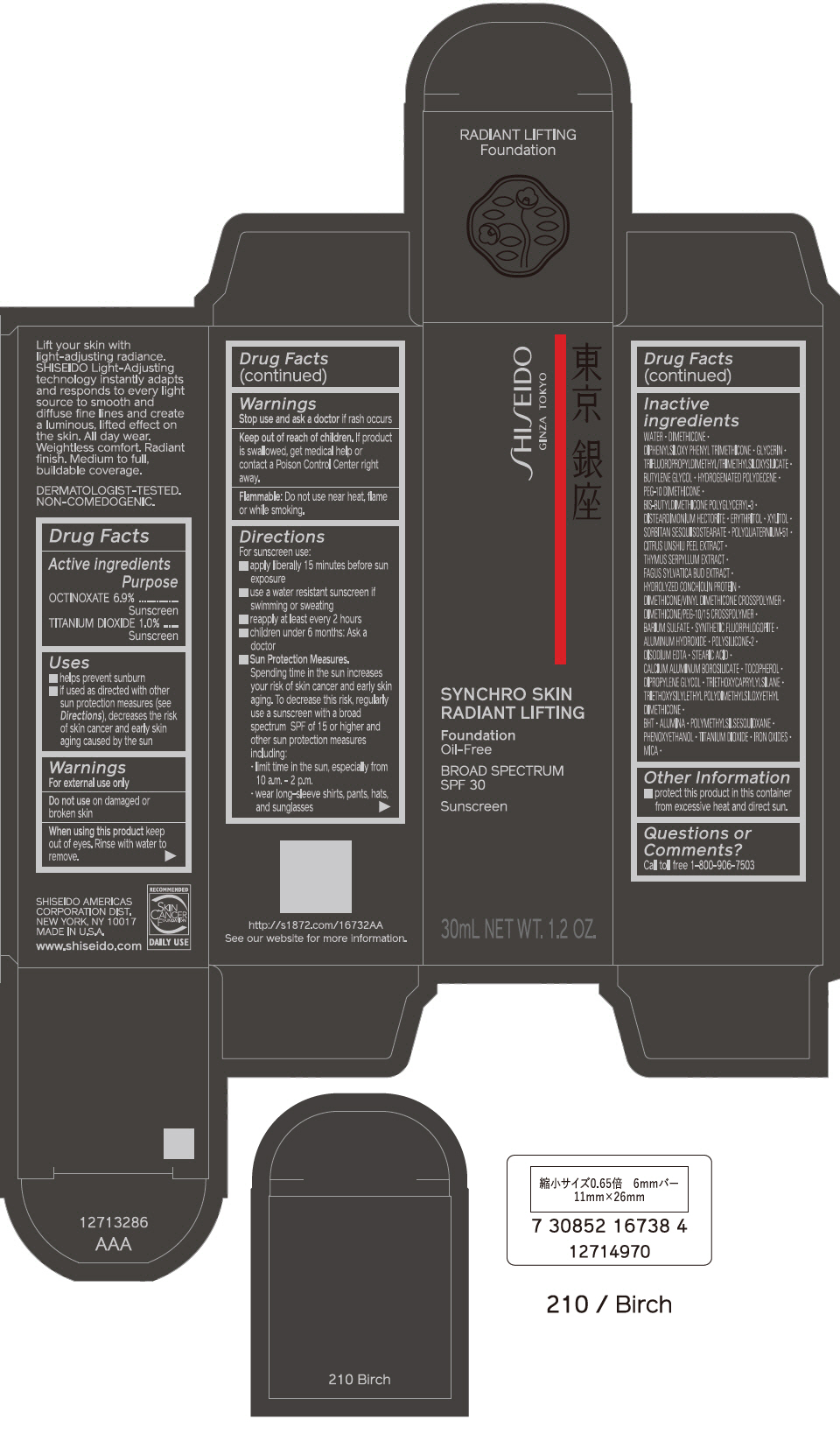
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 220 Linen
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
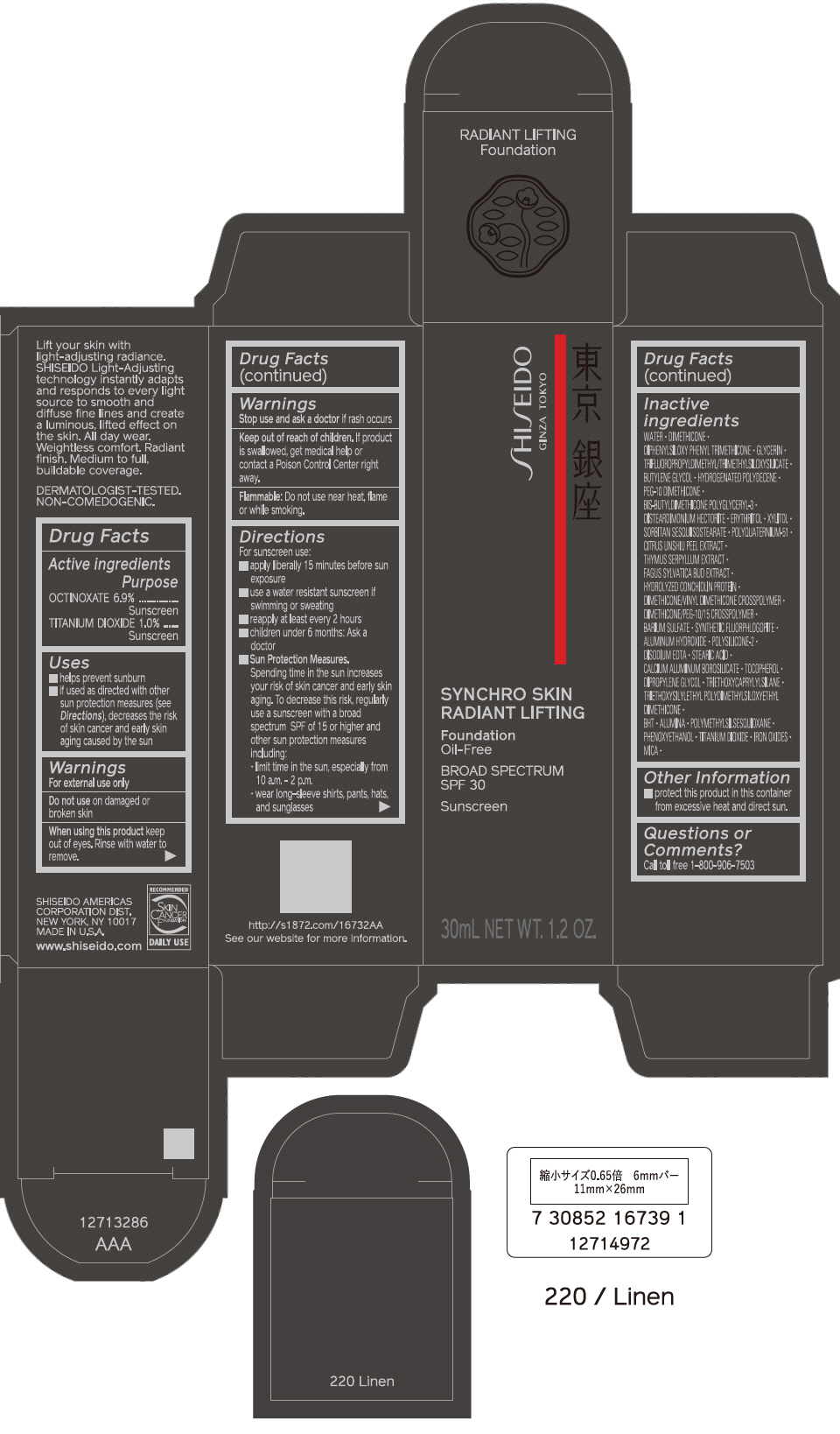
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 230 Alder
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
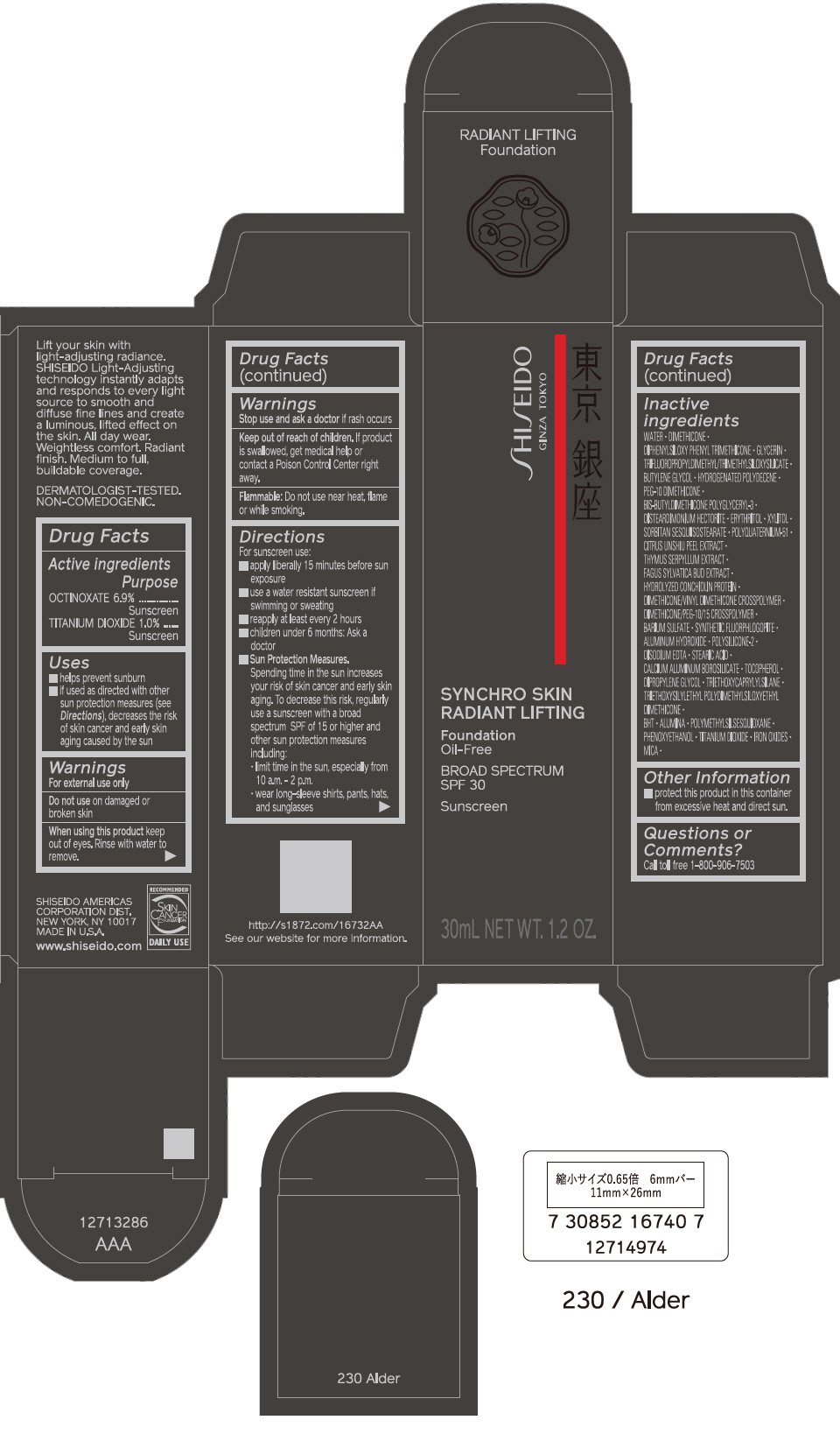
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 240 Quartz
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
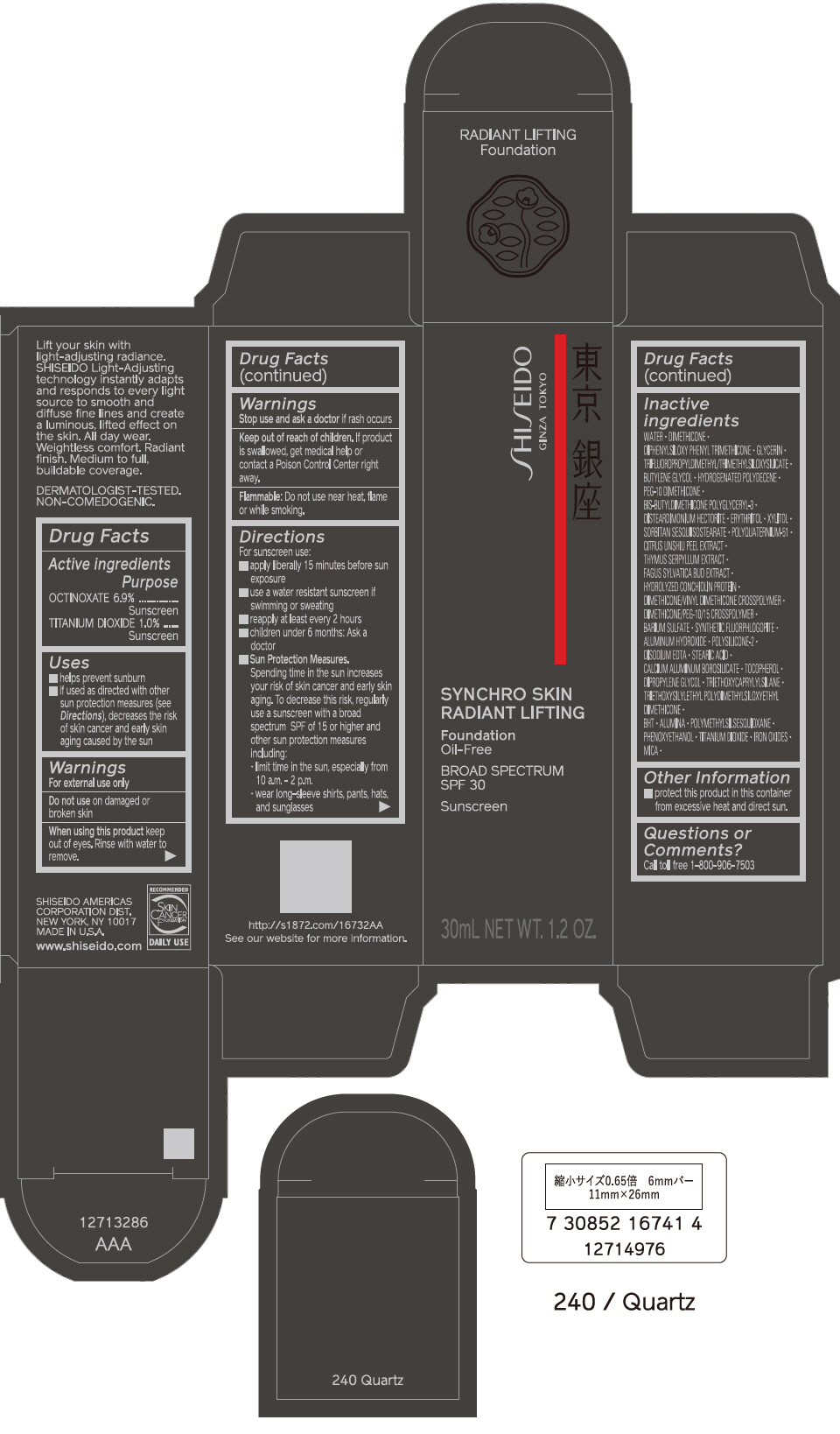
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 250 Sand
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
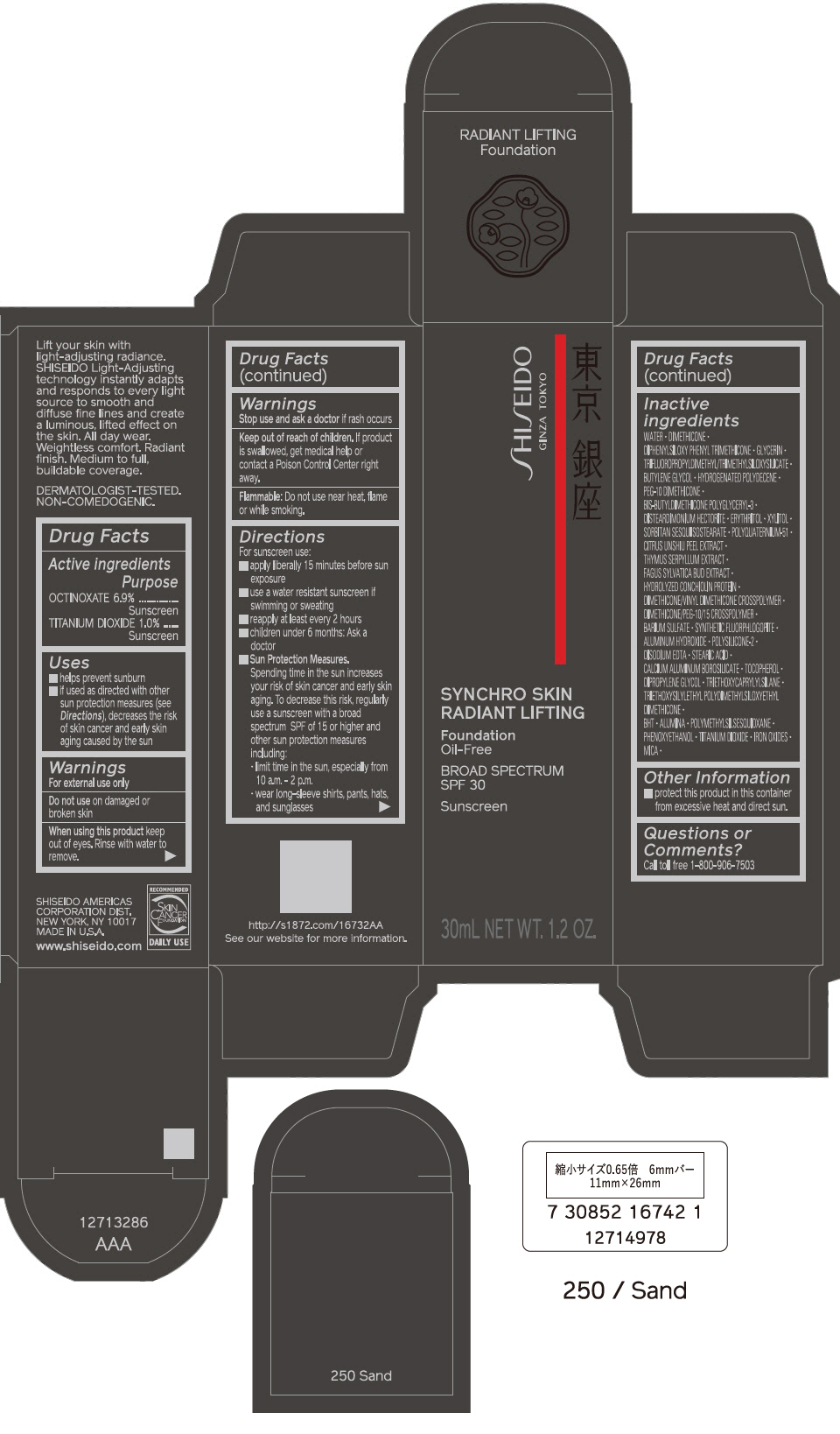
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 260 Cashmere
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
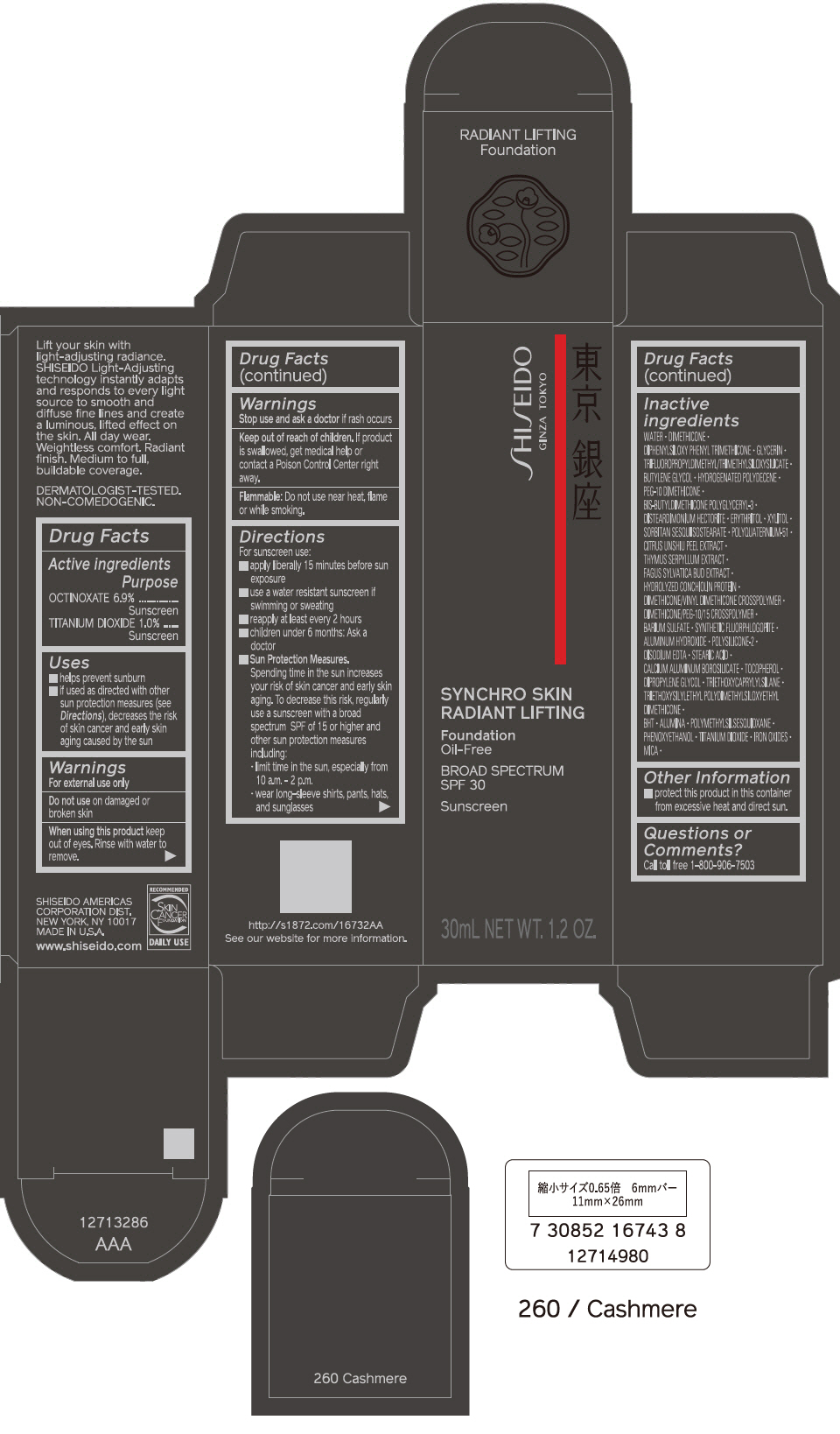
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 310 Silk
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
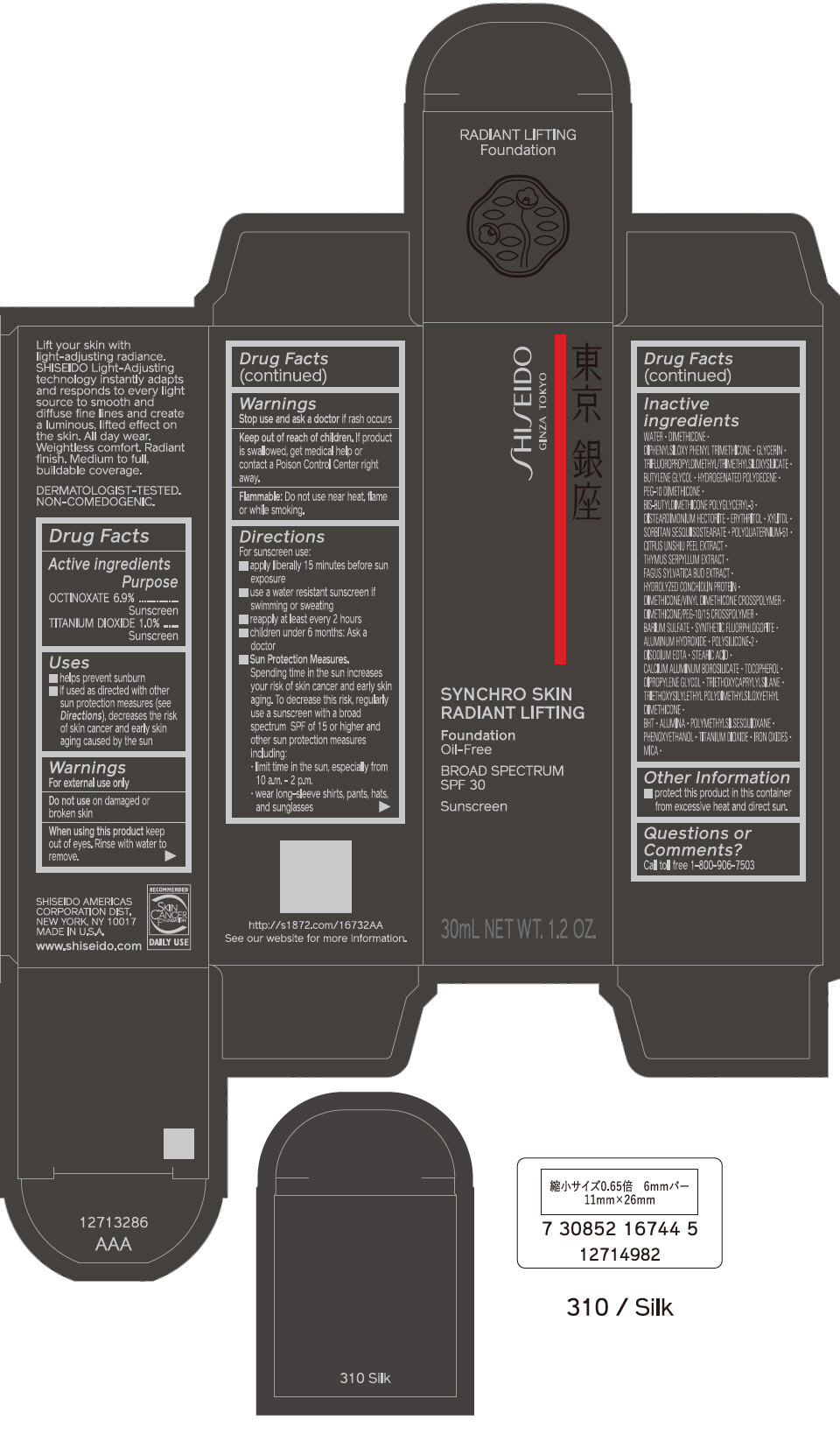
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 320 Pine
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
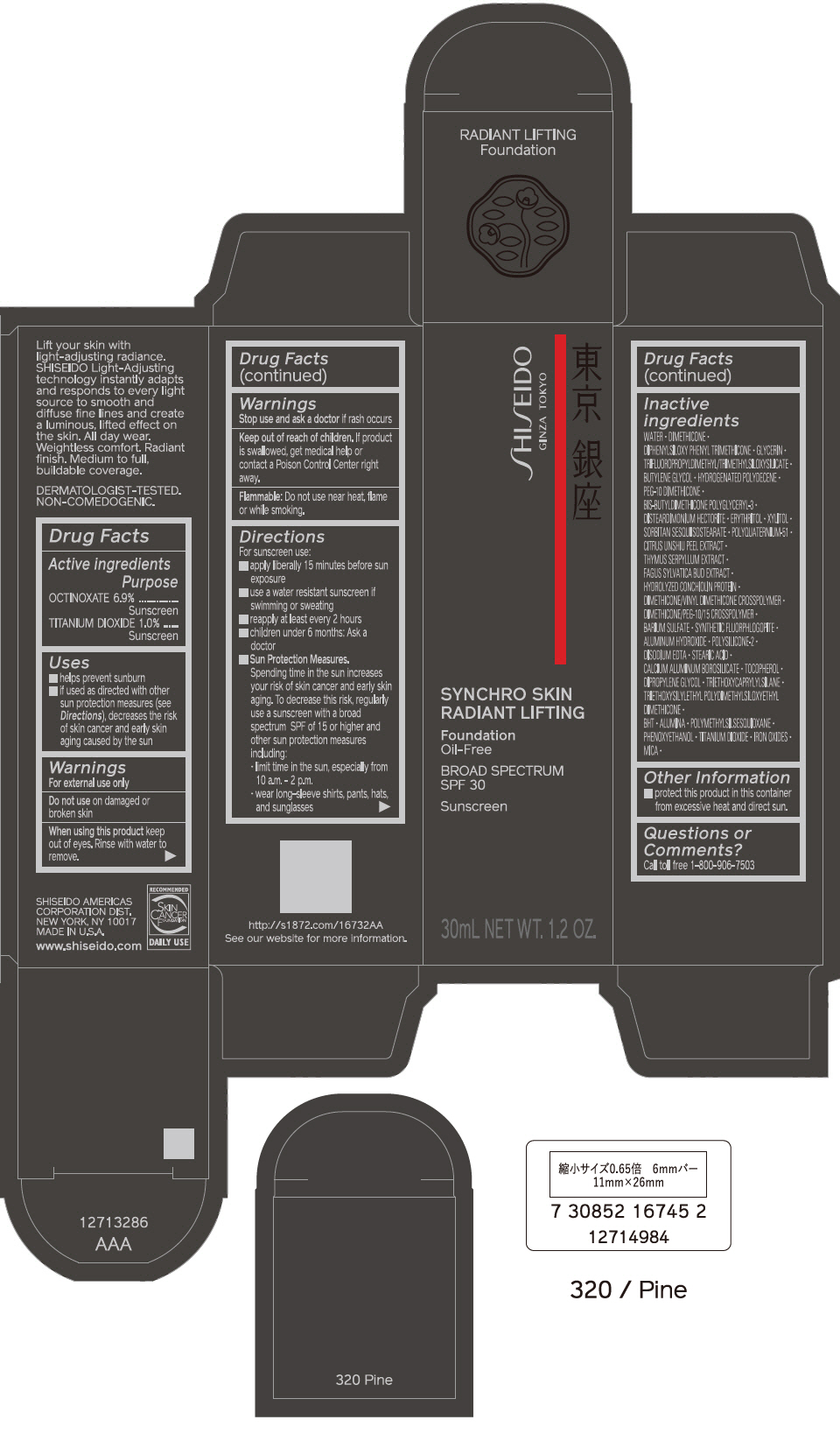
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 330 Bamboo
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
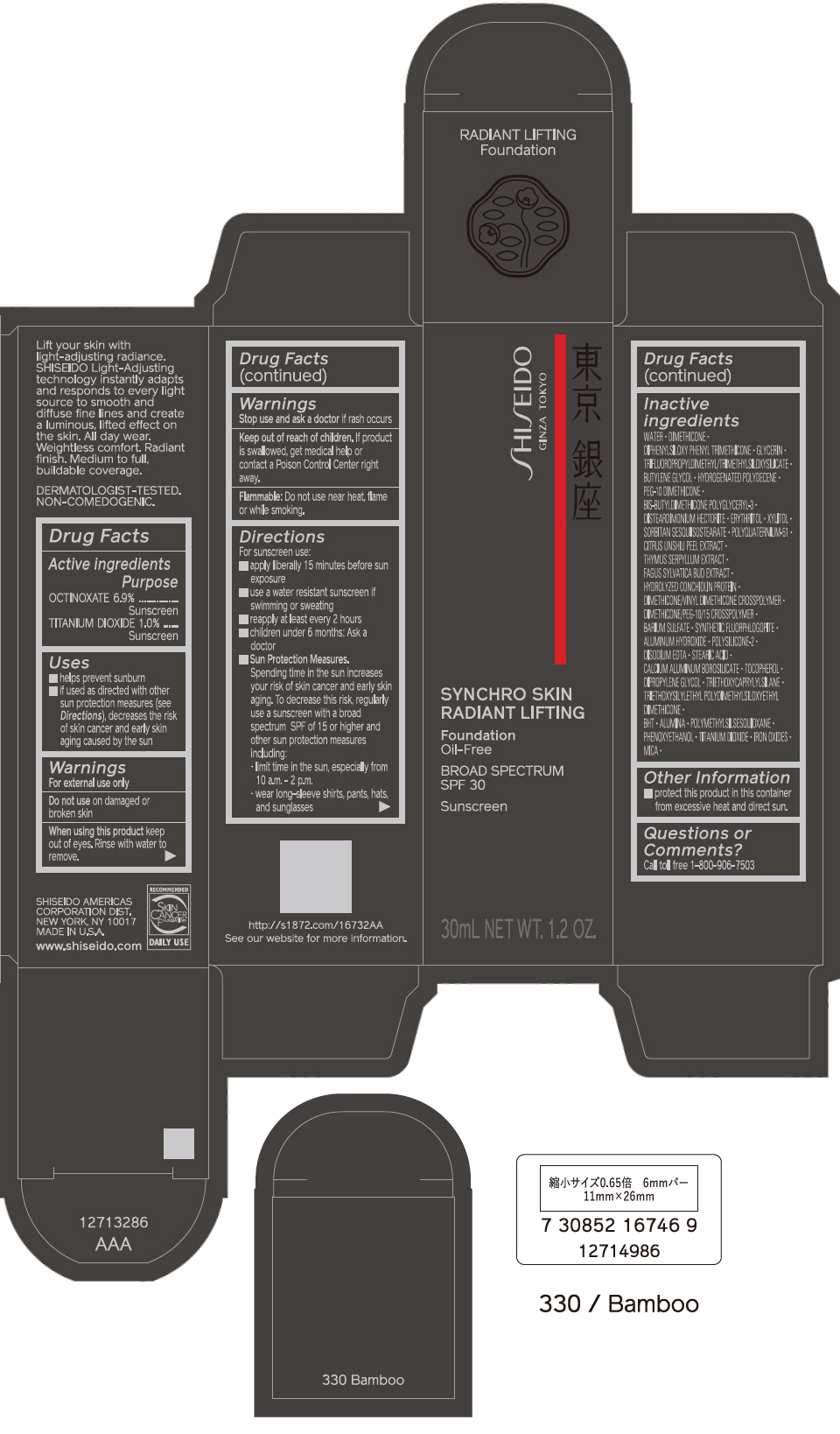
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 340 Oak
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
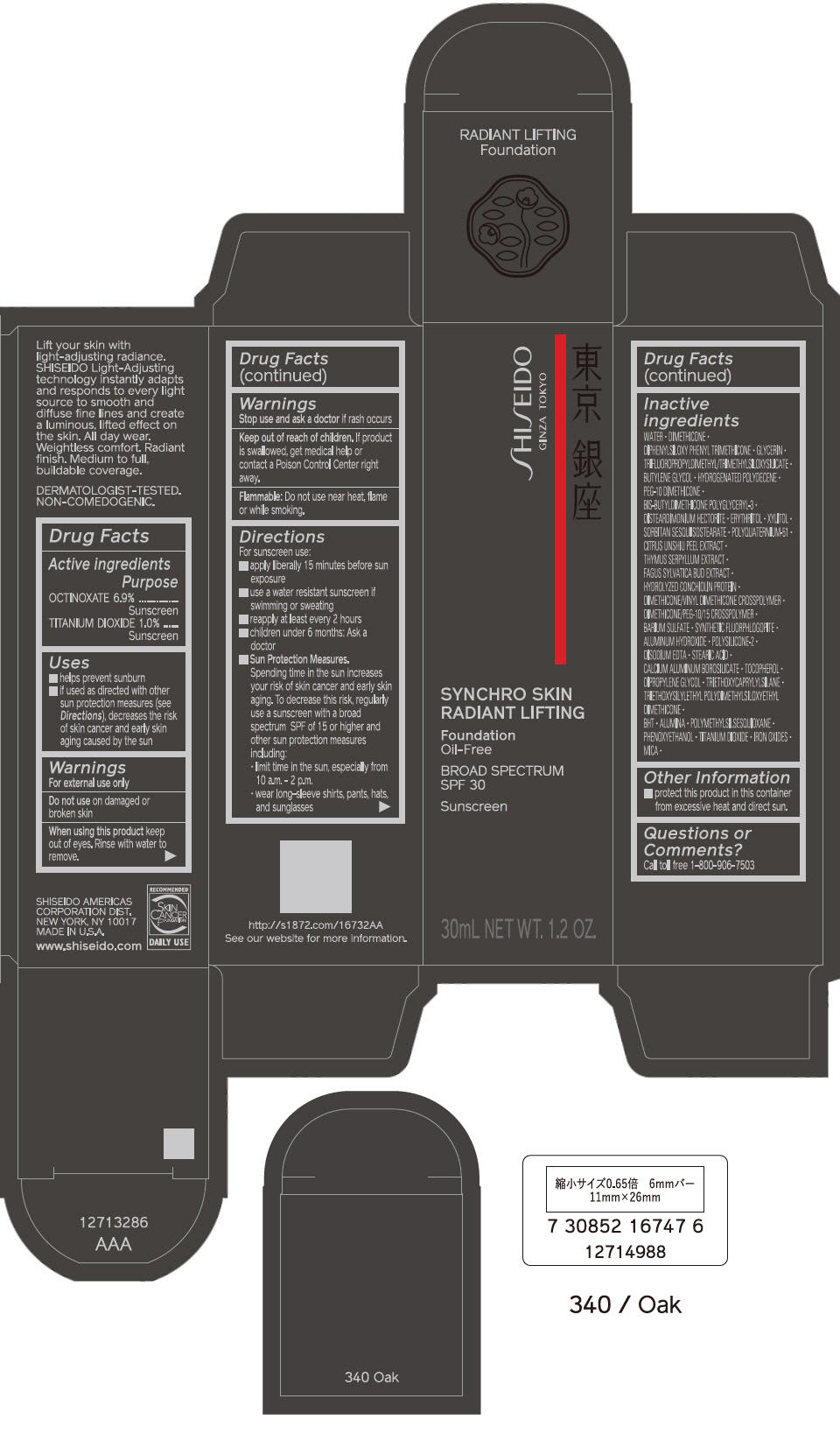
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 350 Maple
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
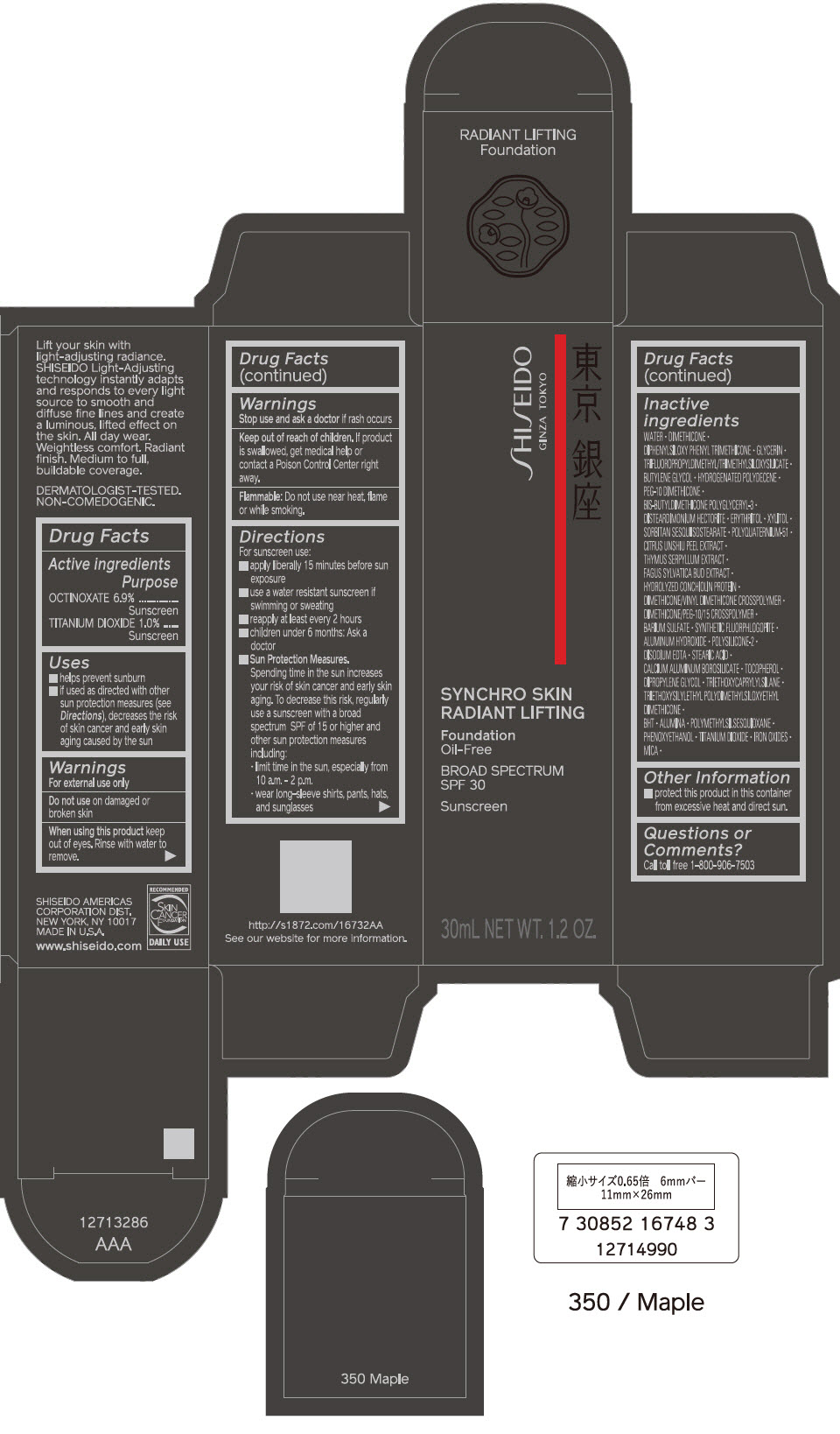
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 360 Citrine
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
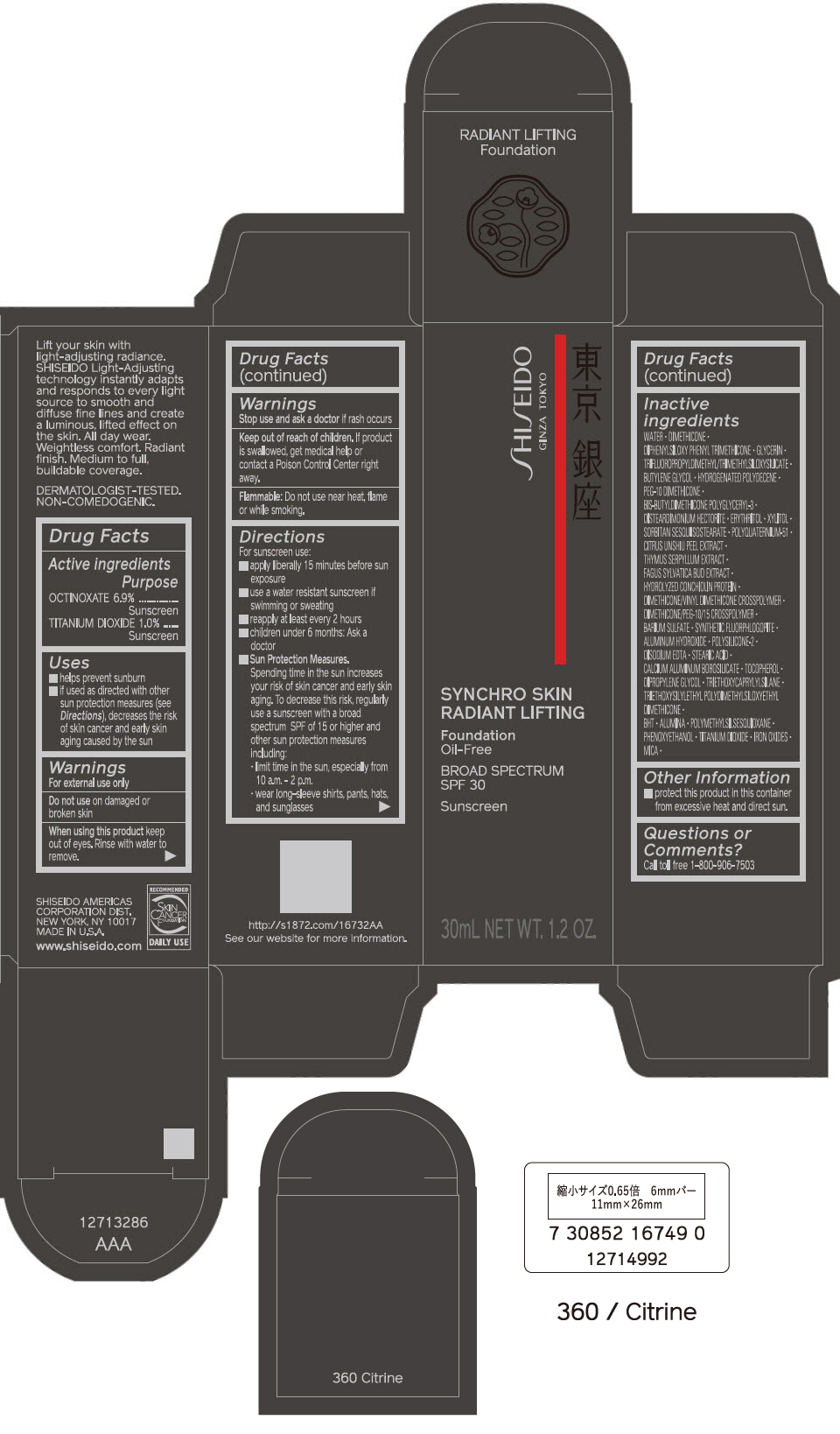
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 410 Sunstone
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
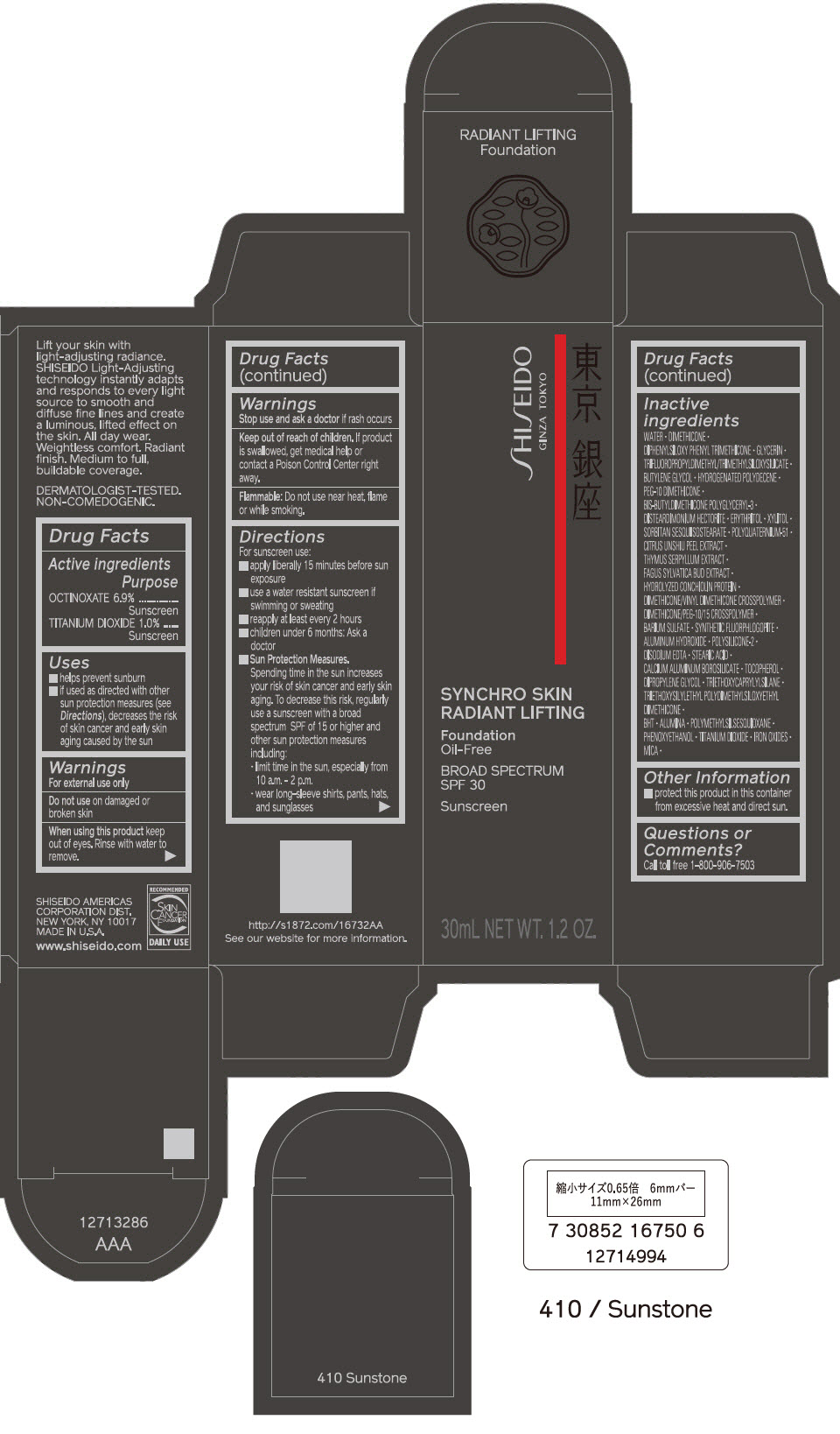
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 420 Bronze
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
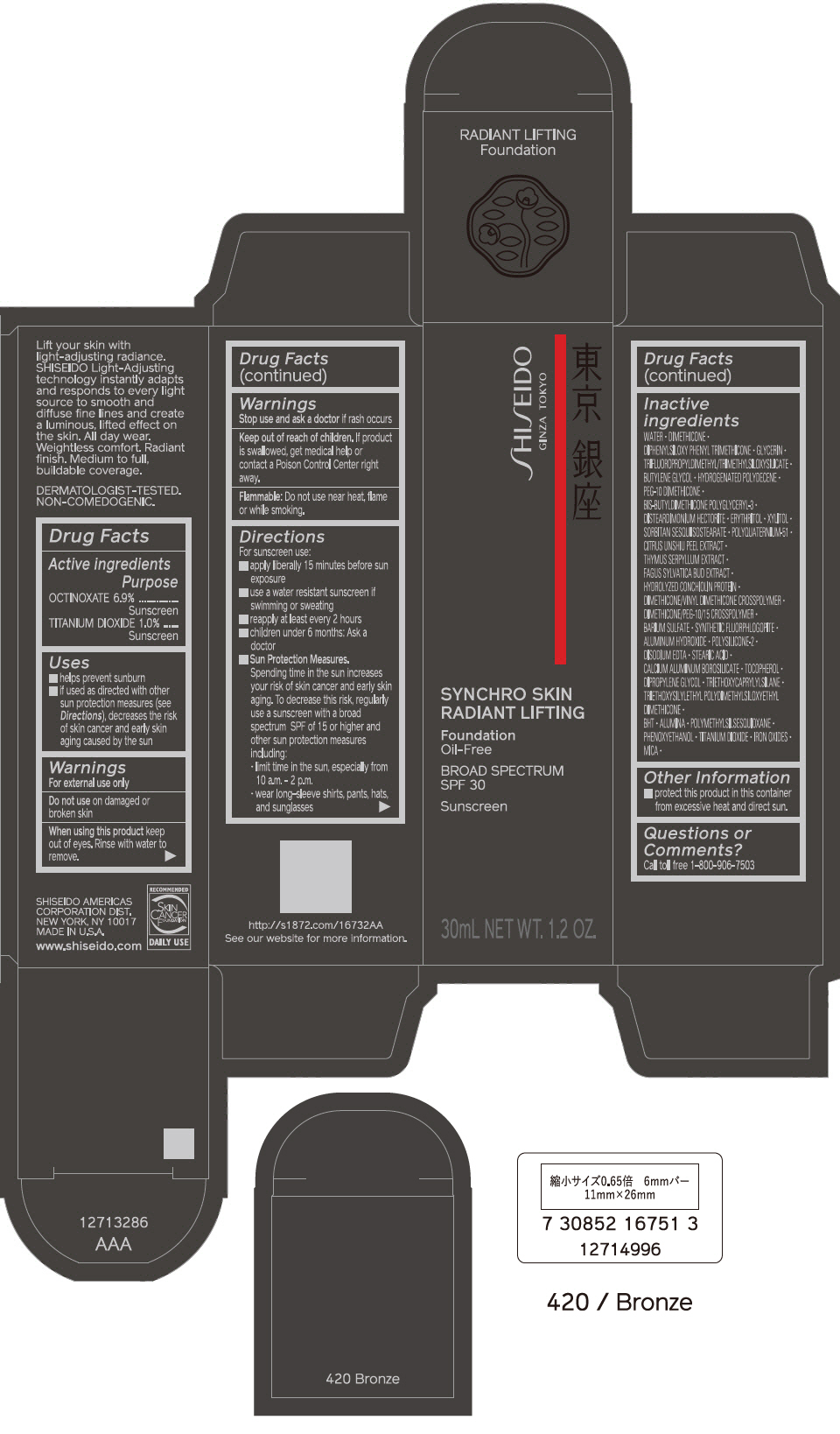
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 430 Cedar
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
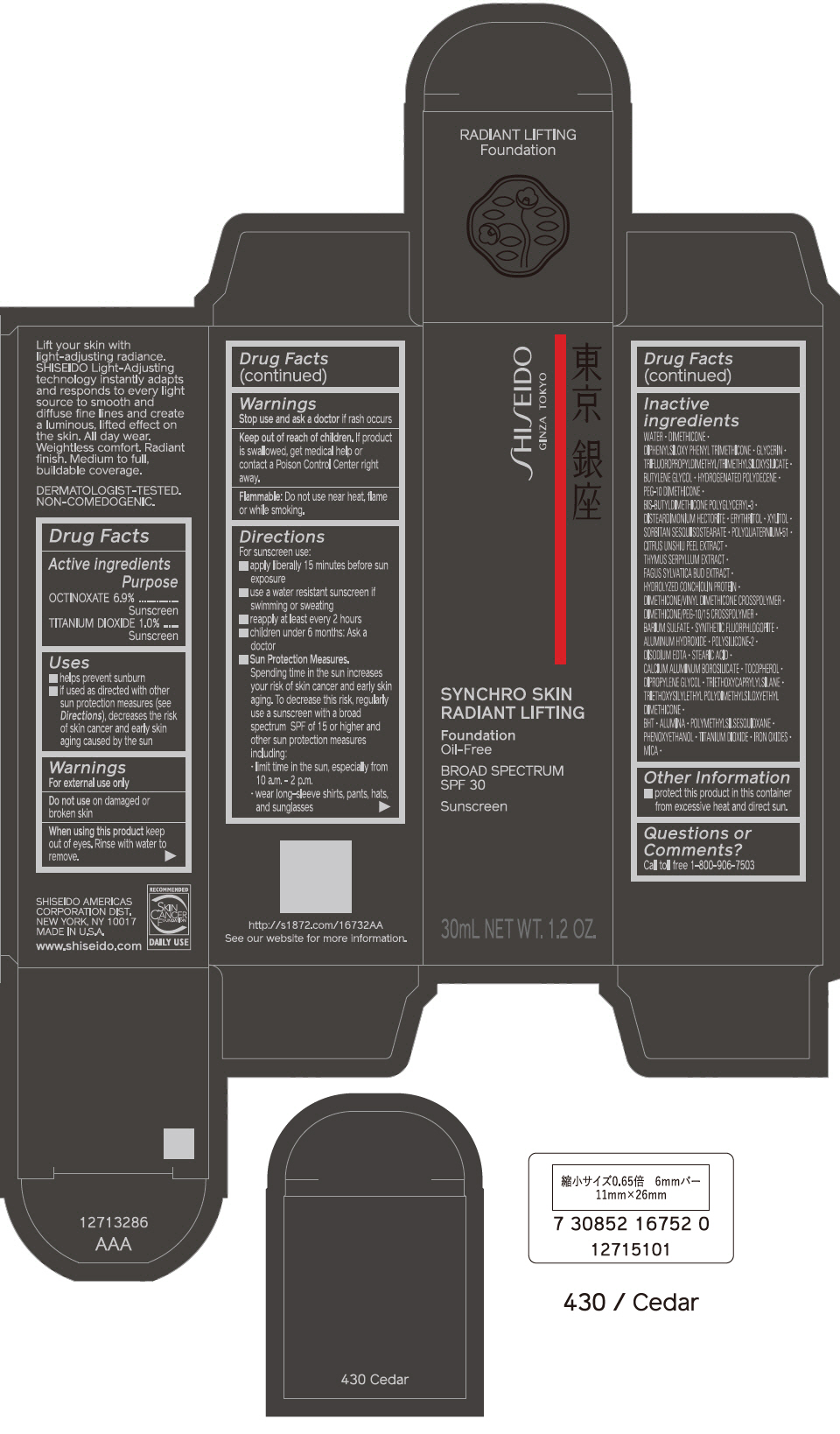
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 440 Amber
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
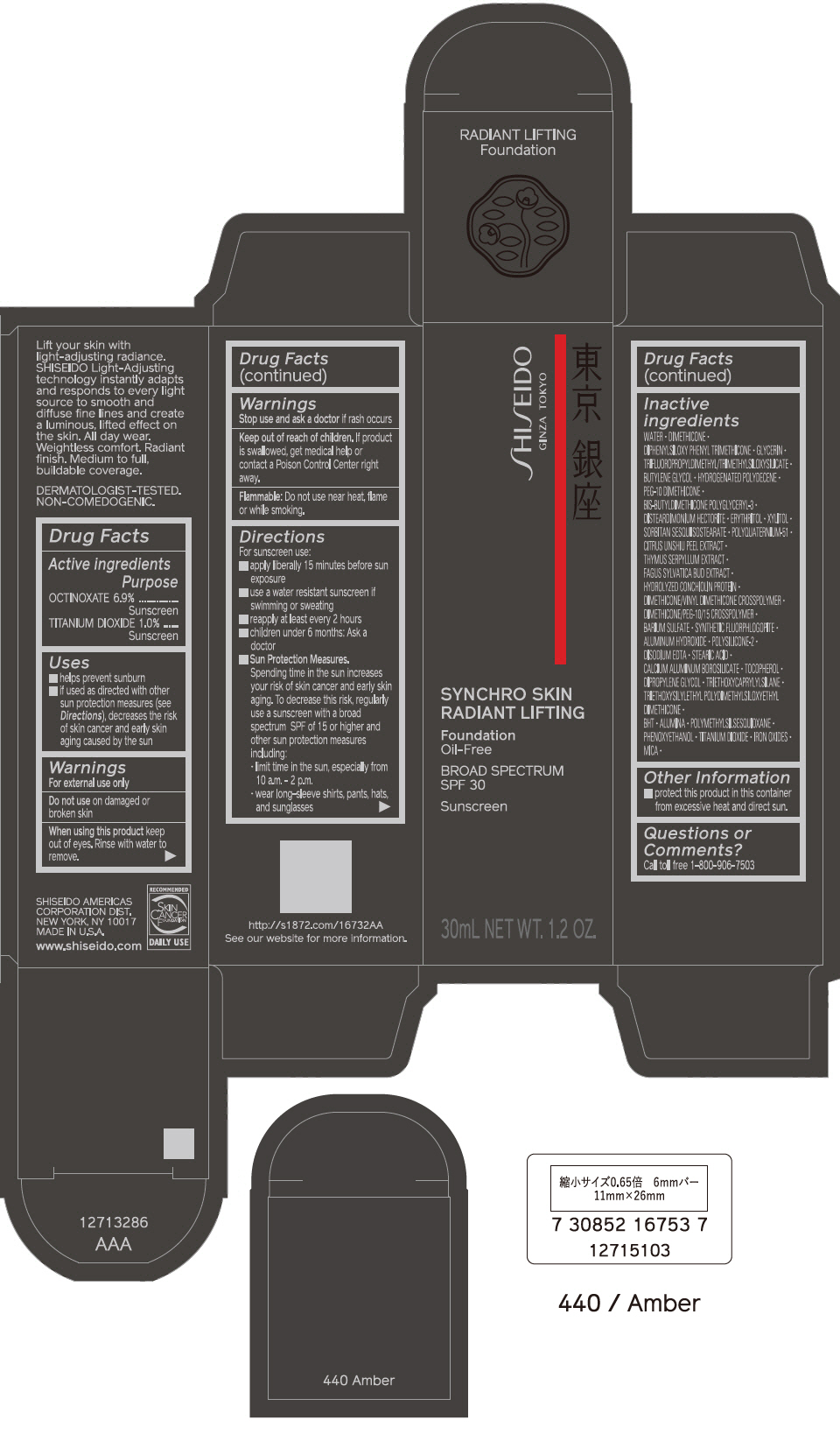
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 450 Copper
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
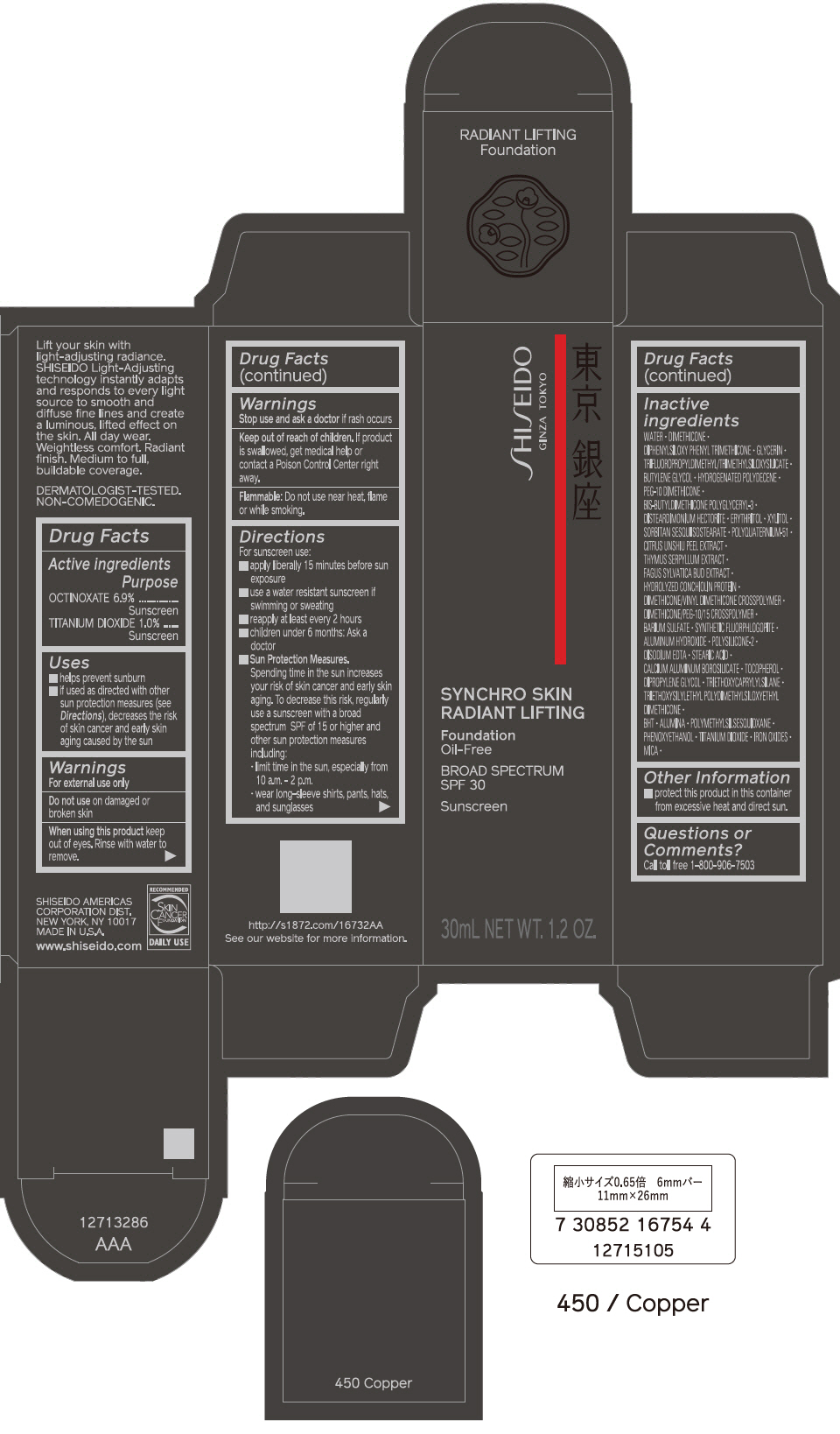
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 460 Topaz
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
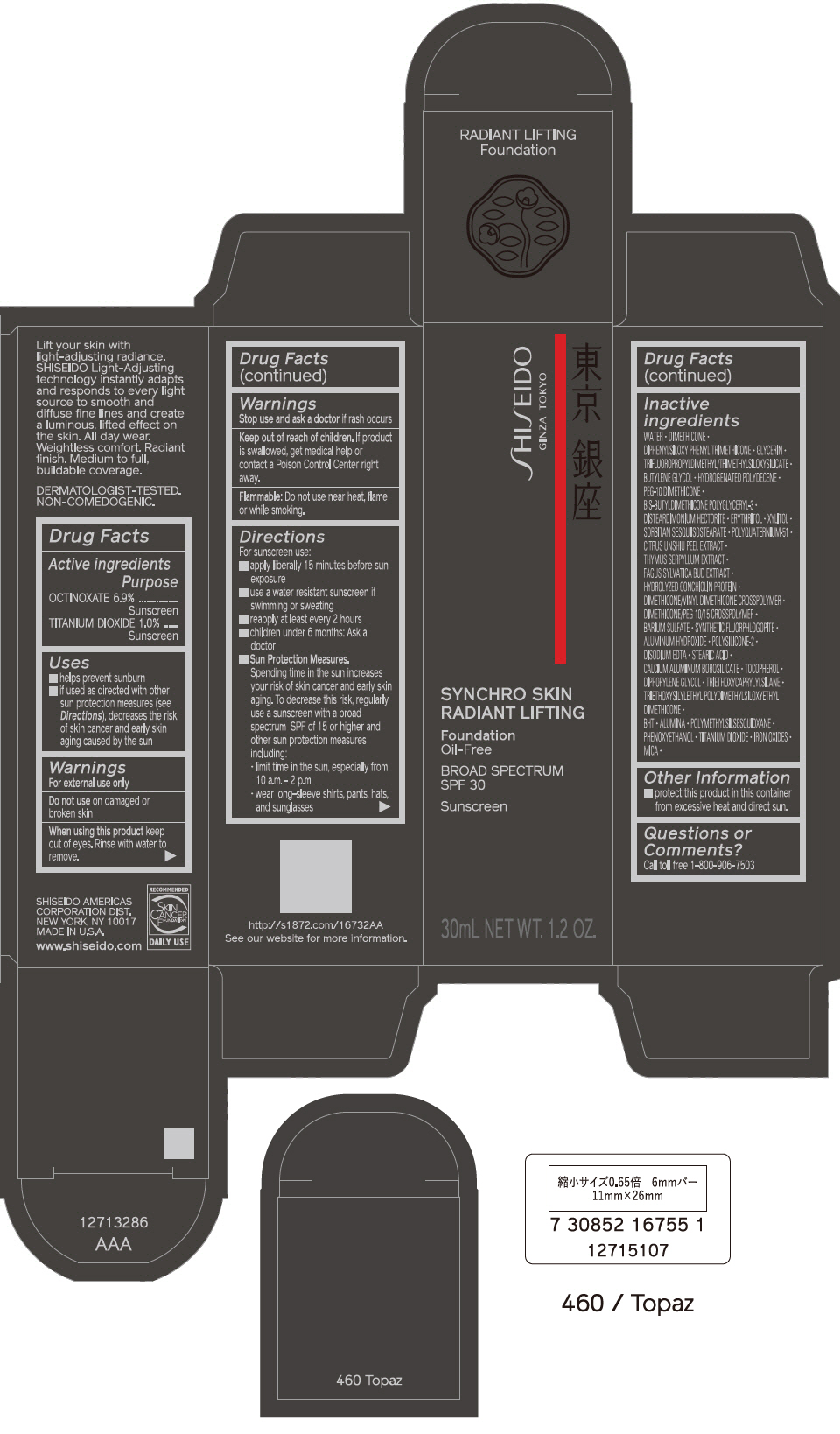
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 510 Suede
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
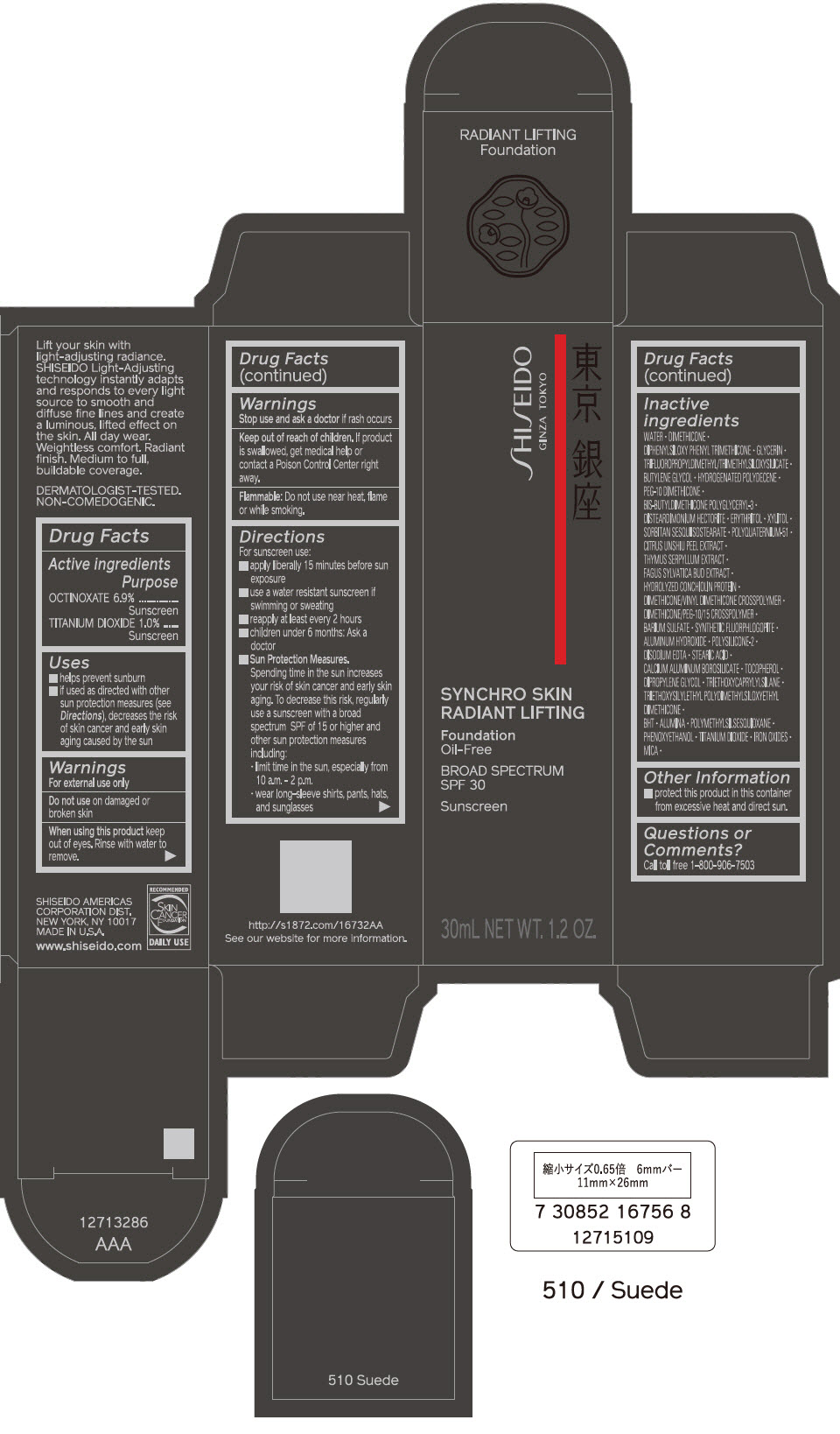
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 520 Rosewood
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
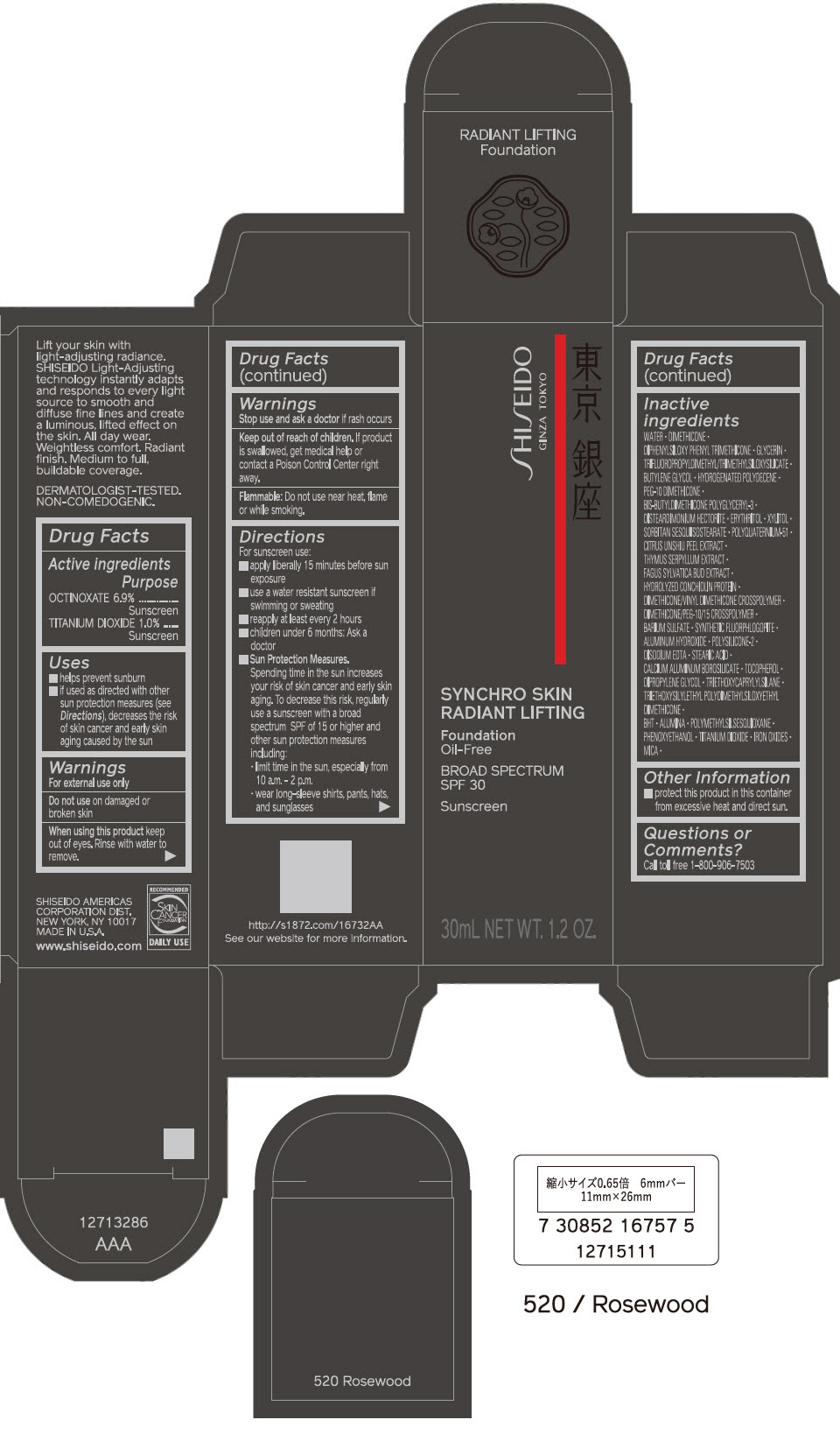
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 530 Henna
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.
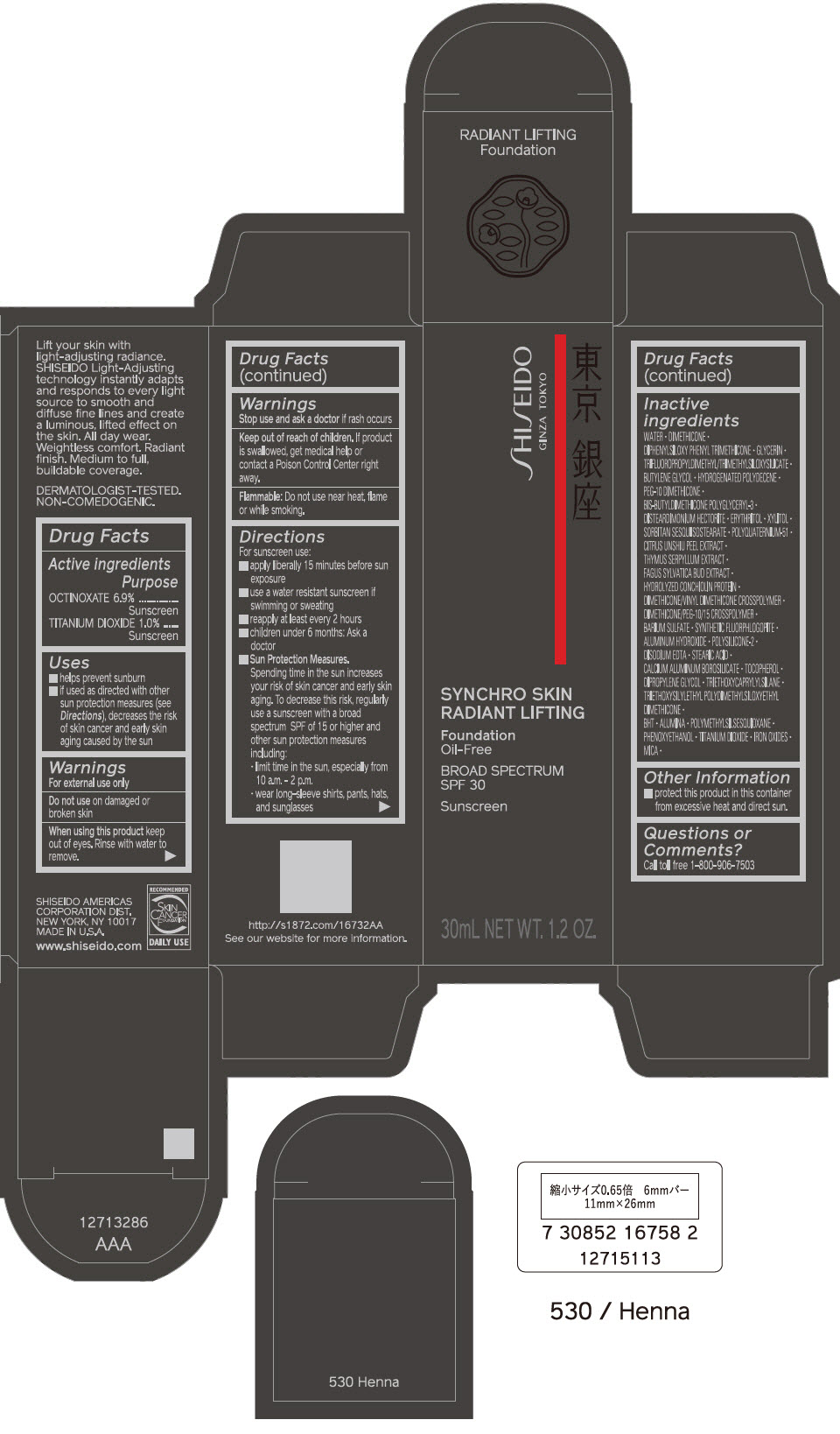
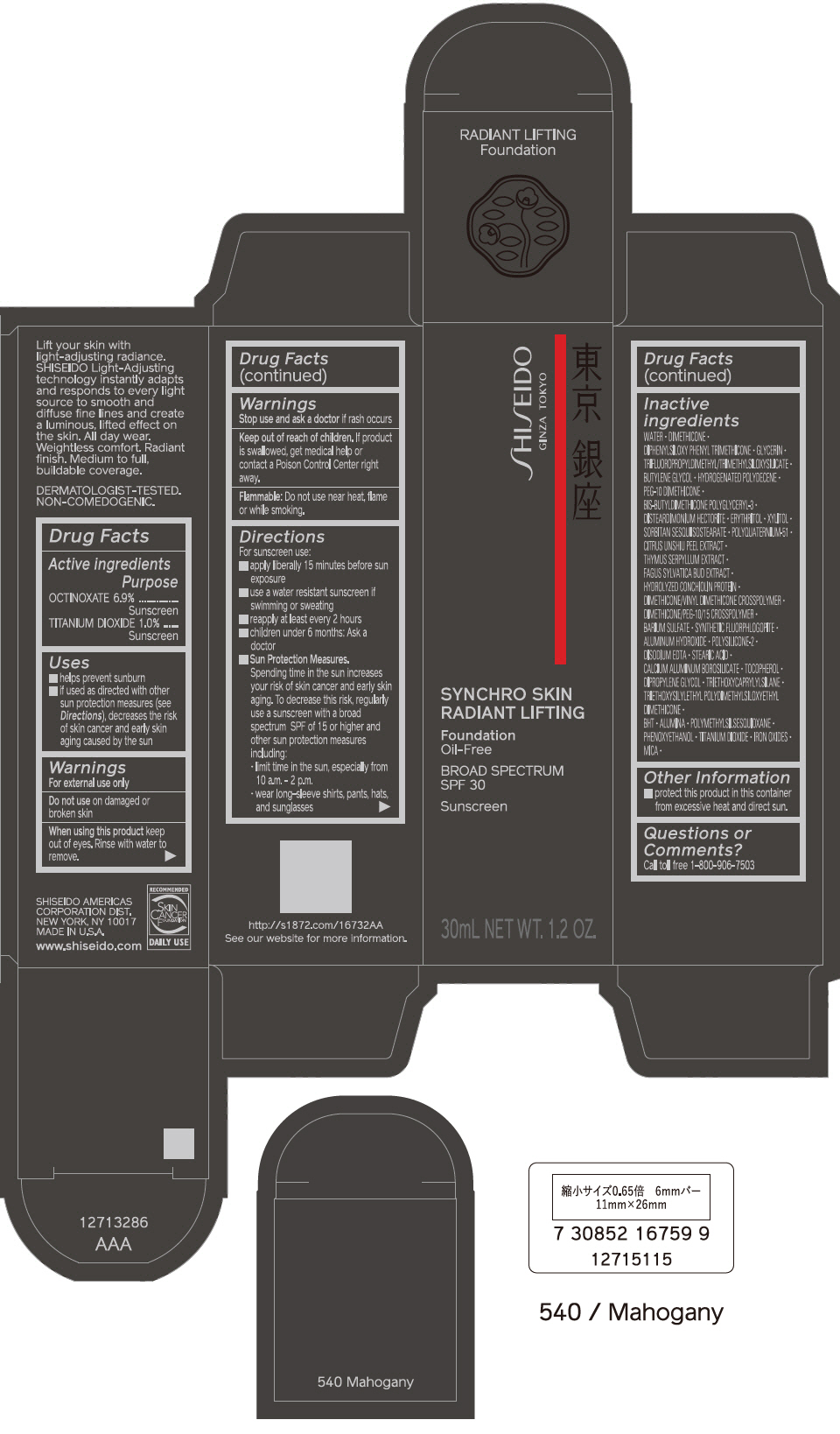
PRINCIPAL DISPLAY PANEL - 30 mL Bottle Carton - 540 Mahogany
SHISEIDO
GINZA TOKYO
SYNCHRO SKIN
RADIANT LIFTING
Foundation
Oil-Free
BROAD SPECTRUM
SPF 30
Sunscreen
30mL NET WT. 1.2 OZ.