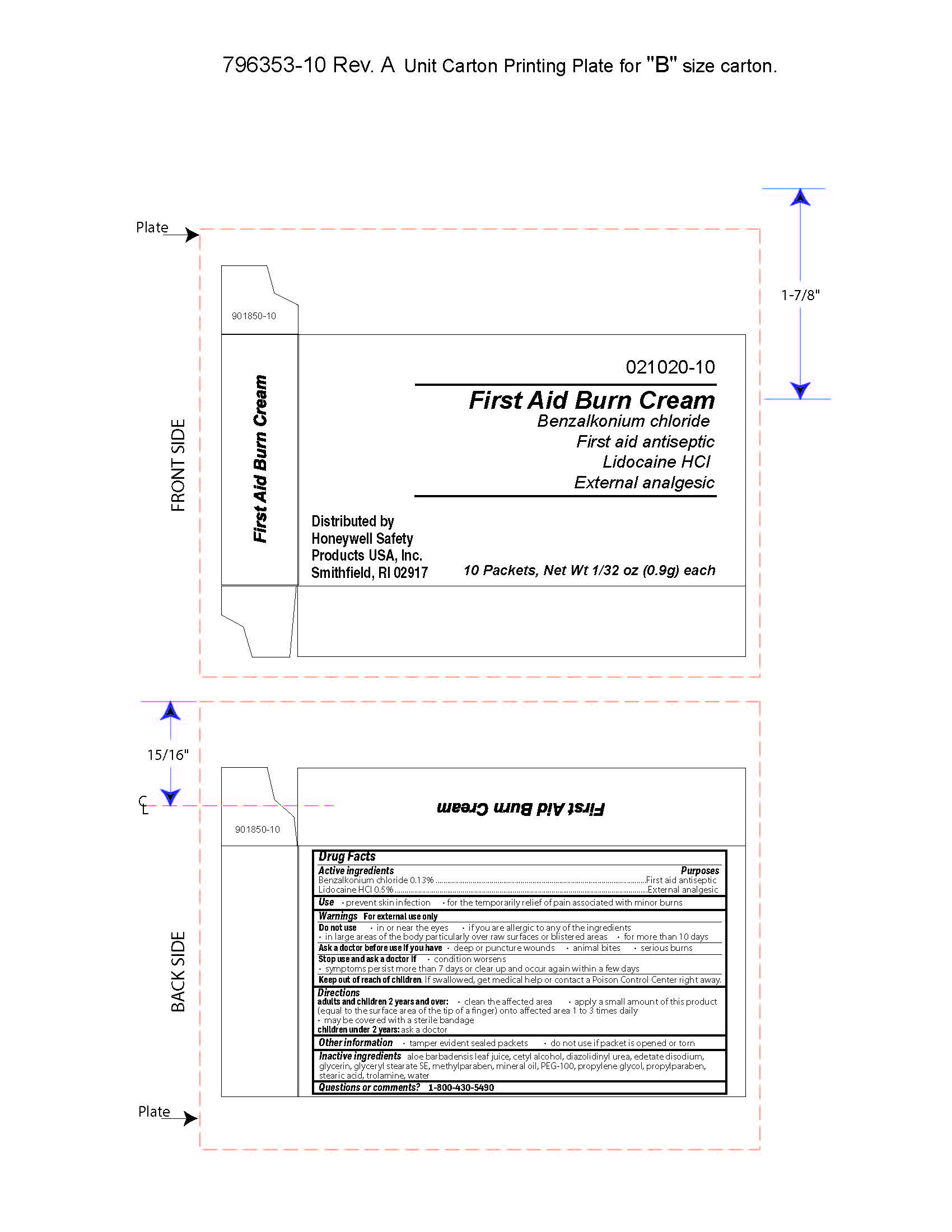
First Aid Burn Cream
Uses
- prevent skin infection
- for temporary relief of pain associated with minor burns
First Aid Burn Cream
Warnings
For external use only
First Aid Burn Cream
Directions
- adults and children 2 years of age and older:
- clean the affected area
- apply a small amount of this product (equal to the surface area of the tip of a finger) onto affected area 1 to 3 times daily
- may be covered with a sterile bandage
- children under 2 years of age: consult a doctor
Inactive ingredients
aloe barbadensis juice, cetyl alcohol, diazolidinyl urea, edetate disodium, glycerin, glyceryl stearate SE, methylparaben, mineral oil, PEG-100, propylene glycol, propylparaben, stearic acid, trolamine, water
Neomycin Antibiotic Ointment
Active ingredient
Neomycin sulfate (5 mg equivalent to 3.5 mg Neomycin base)
Neomycin Antibiotic Ointment
Uses
first aid to help prevent infection in - minor cuts - scrapes - burns
Neomycin Antibiotic Ointment
Warnings
For external use only
Neomycin Antibiotic Ointment
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
PVP
Warnings
For external use only
PVP
Directions
- clean the affected area
- apply1 to 3 times daily
- may be covered with a sterile bandage
- if bandaged, let dry first
- discard wipe after single use
PVP
Other information
- do not use on individuals who are allergic or sensitive to iodine
- store at controlled temperature 59-86ºF (15-30ºC)
- do not use if pouch is open or torn
4351
018503-4219 Kit Contents
1 TWEEZER PLASTICS 4"
2 O/H PAK,ADH BDG 2"X4", X-LG
6 PVP PREP PADS MEDIUM
1 SCISSOR BDGE 4" RED PLS HDL
1 PR LRG NITRILE GLVES ZIP BAG
4 FIRST AID CREAM 1.0GR PKT EACH
1 TAPE ADHESIVE 1/2 X 2.5 125133
4 POUCH NEOMYCIN ANTIBIOTIC .9 G
1 KIT BAG SOFT PACK PROMO
1 INSRT POCKET KIT PROD INF ID C
1 COLD PACK UNIT 4"X6" BULK
2 GAUZE PADS 3"X3" 12PLY
2 WOVEN FINGERTIP BANDAGE 2"
2 WOVEN KNUCKLE BANDAGE
10 WOVEN BANDAGE 1" X 3"