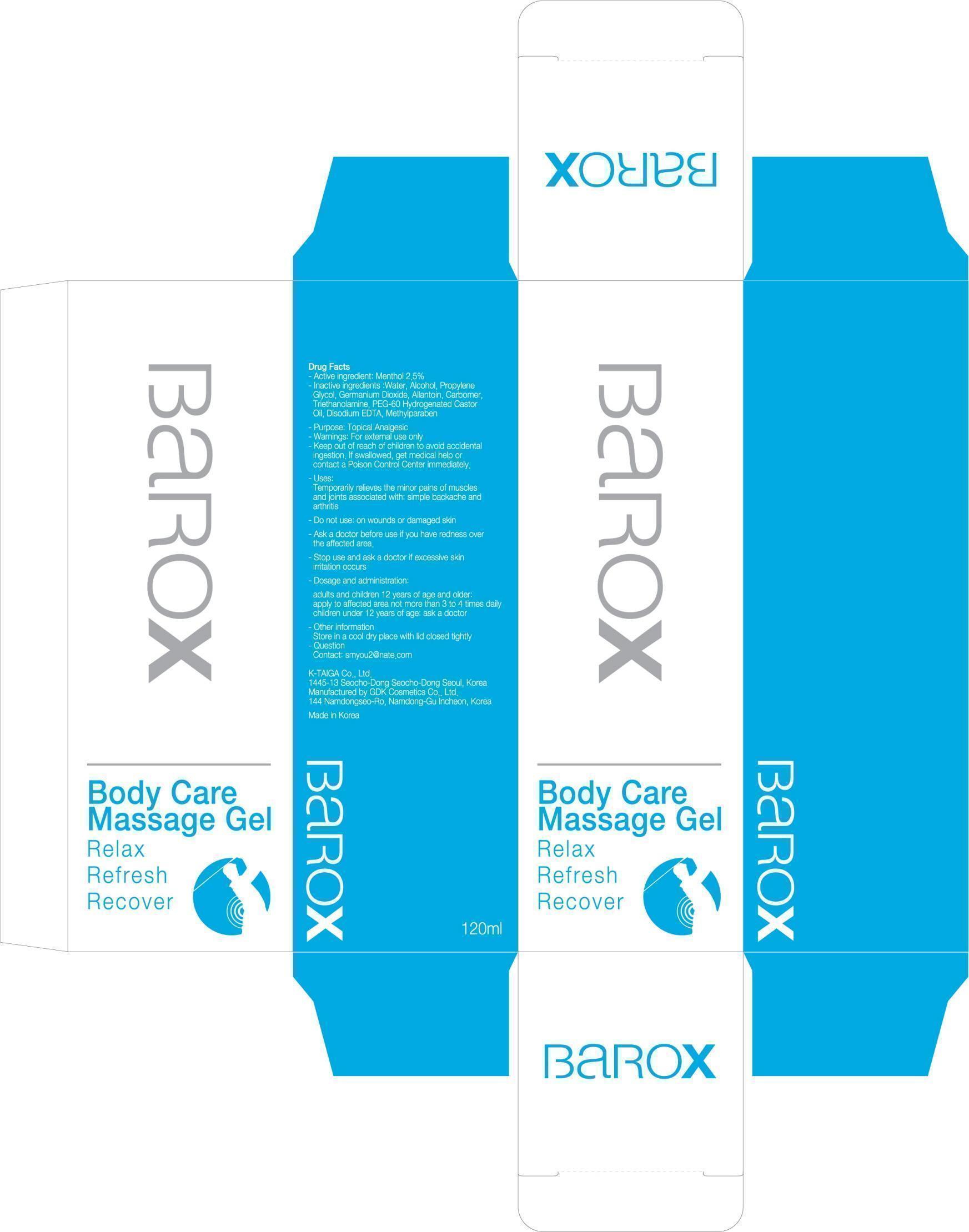
Inactive ingredient
Inactive ingredients:
Water, Alcohol, Propylene Glycol, Germanium Dioxide, Allantoin, Carbomer, Triethanolamine, PEG-60 Hydrogenated Castor Oil, Disodium EDTA, Methylparaben
Keep out of reach of children
Keep out of reach of children:
Keep out of reach of children to avoid accidental ingestion.
If swallowed, get medical help or contact a Poison Control Center immediately.
Uses
Uses:
Temporarily relieves the minor pains of muscles and joints associated with:
simple backache and arthritis
Dosage and administration
Dosage and administration:
adults and children 12 years of age and older: apply to affected area not more than 3 to 4 times daily
children under 12 years of age: ask a doctor
Description
Ask a doctor before use if you have redness over the affected area.
Do not use: on wounds or damaged skin
Stop use and ask a doctor if excessive skin irritation occurs.
Other information:
Store in a cool dry place with lid closed tightly
Question
Contact: smyou2@nate.com