Eyewash
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyewash
Warnings
For external use only Obtain immediate medical treatment for all open wounds in or near eyes. To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Eyewash
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
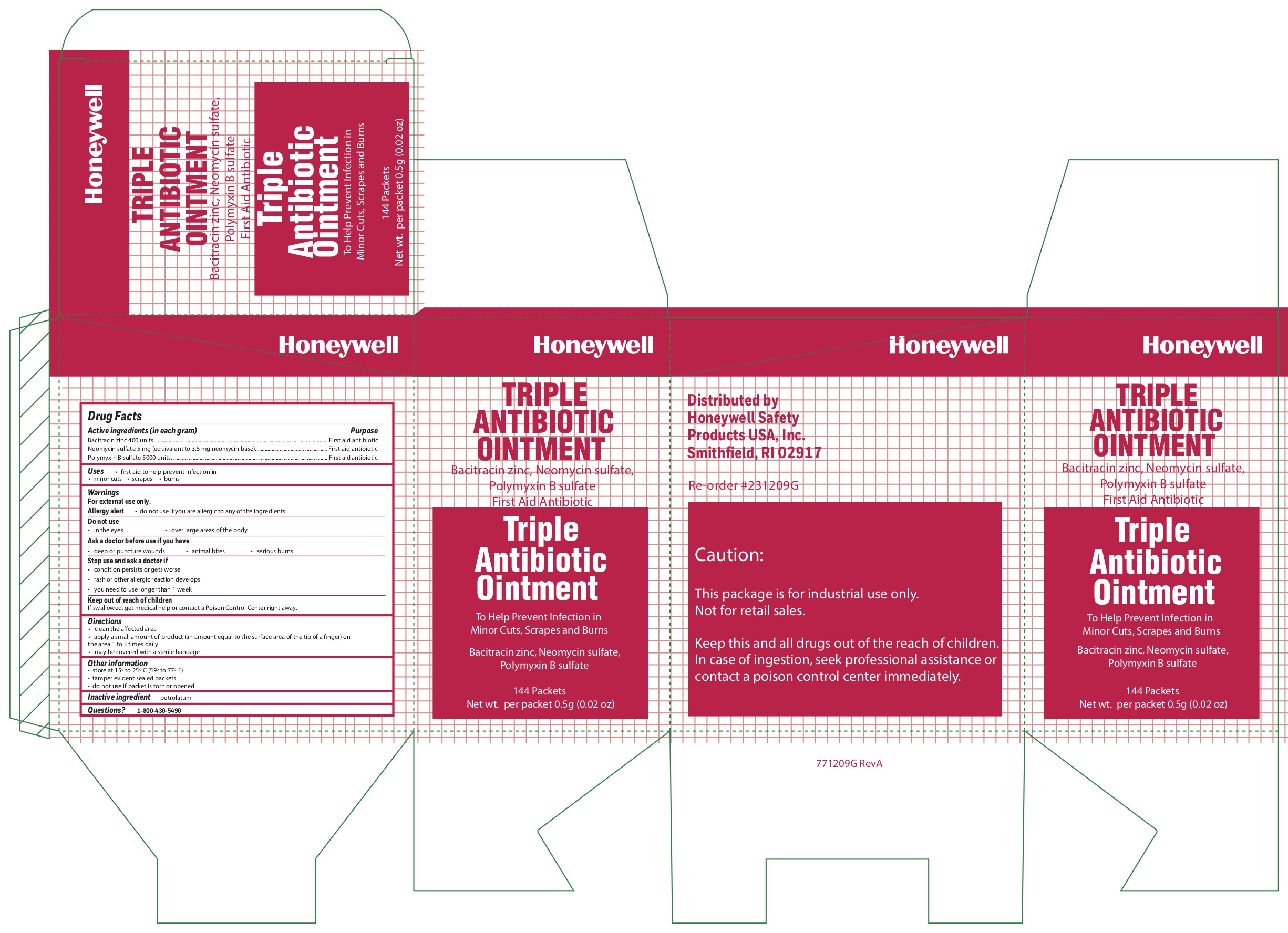
Triple
Active ingredients
Bacitracin zinc 400 units
Neomycin sulfate (5 mg equivalent to 3.5 mg Neomycin base)
Polymyxin B sulfate 5000 units
Triple
Warnings
For external use only
Allergy alert do not use if you are allergic to any of the ingredients
Triple
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Triple
Other information
- store at 15 0 to 25 0 C (59 0 to 77 0 F)
- tamper evident sealed packets
- do not use if packet is torn or opened
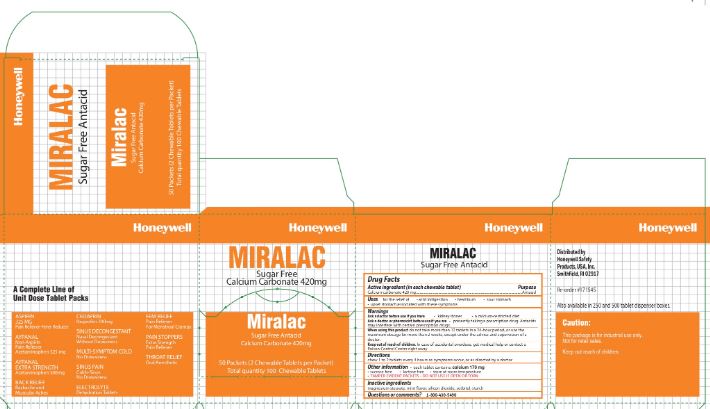
Miralac
Uses
for the relief of
- acid indigestion
- heartburn
- sour stomach
- upset stomach associated with these symptoms
Warnings
Ask a doctor before use if you have
- kidney stones
- calcium-restricted diet
Ask a doctor before use if you are
- presently taking a prescription drug. Antacids may interfere with certain prescription drugs
Miralac
Other information
- each tablet contains: calcium 170 mg
- sucrose free
- lactose free
- store at room temperature
- TAMPER EVIDENT PACKETS- DO NOT USE IF OPEN OR TORN
BZK
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
BZK
Other information
- store at room temperature 15 0 to 30 0 C (5 0 - 86 0 F)
- do not reuse towelette
Pain Stopper
Active ingredient (in each tablet)
Acetaminophen 110mg
Aspirin 162mg (NSAID)*
Caffeine 32.4mg
Salicylamide 152mg (NSAID)*
*nonsteroidal anti-inflammatory drug
Pain Stopper
Purpose
Pain reliever/fever reducer
Pain reliever/fever reducer
Diuretic
Pain reliever/fever reducer
Pain Stopper
Uses
for the temporary relief of minor aches and pains due to:
• common cold
• headache
• muscular aches
• premenstrual and menstrual cramps
Pain Stopper
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If skin reaction occurs, stop use and seek medical help right away.
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 12 tablets in 24 hours, which is the maximum daily amount
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist
- for more than 10 days for pain unless directed by a doctor
- for more than 3 days for fever unless directed by a doctor
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you are taking a diuretic
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
Stop using and ask a doctor if
- symptoms do not improve
- new symptoms occur
- pain or fever persists or gets worse
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pin that does not get better
- if ringing in the ears or a loss of hearing occurs, consult a doctor before taking any more of this product.
Pain Stopper
Directions
- adults and children 12 years of age and over, take 2 tablets every 4 hours while symptoms persist
- do not take more than 12 tablets in 24 hours
- children under 12 years: consult a doctor
Pain Stopper
Other information
- store at a controlled room temperature 15 0 -30 0 C (59 0 -86 0 F)
- TAMPER EVIDENT-DO NOT USE IF OPEN OR TORN
Pain Stopper
Inactive ingredients
FD&C Yellow #6, magnesium stearate, microcrystalline cellulose, povidone, starch, stearic acid,
Burn Relief WJ
Warnings
For external use only
Burn Relief WJ
Directions
- adults and children 2 years of age and older: apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
Burn Relief WJ
Inactive ingredients
diazolidinyl urea, edetate disodium, glycerin, hypromellose, methylparaben, octoxynol 9, propylene glycol, propylparaben, purified water, tea tree oil, trolamine
4346
Z68140GRR KIT CONTENTS
1 1X3 PLASTIC 100/BOX
1 WOVEN 7/8 X 3 50/BOX
1 SWIFT KNUCKLE 40/BX
1 SWIFT FINGERTIP 8 50/BOX
2 TRIPLE ANTIBIOTIC 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
1 INSTANT COLD PACK 4" X 6"
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 TWEEZER PLASTICS 4"
1 O/H PUMP BURN RELIEF 2 OZ ID G
1 BLOODSTOPPER
1 NON-ADHERENT PADS 2"X3" 10'S
1 GZE PADS STERILE 3"X 3" 25'S
2 ANTISEPTIC WIPES BZK CHL 20'S
1 PAIN STOPPERS IND PK 2ENV 100
1 MIRALAC TABS IND PK 2/ENV 100
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 F A KIT EMPTY BLANK 140
1 POCKET INSERT RED #140 KIT 2R
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
2 PR LRG NITRILE GLVES ZIP BAG
1 TRI BNDG NON WOVEN 40"X40"X56"