DESCRIPTION
CIPRODEX® (ciprofloxacin 0.3% and dexamethasone 0.1%) Sterile Otic Suspension contains the synthetic broad-spectrum antibacterial agent, ciprofloxacin hydrochloride, combined with the anti-inflammatory corticosteroid, dexamethasone, in a sterile, preserved suspension for otic use. Each mL of CIPRODEX® Otic contains ciprofloxacin hydrochloride (equivalent to 3 mg ciprofloxacin base), 1 mg dexamethasone, and 0.1 mg benzalkonium chloride as a preservative. The inactive ingredients are boric acid, sodium chloride, hydroxyethyl cellulose, tyloxapol, acetic acid, sodium acetate, edetate disodium, and purified water. Sodium hydroxide or hydrochloric acid may be added for adjustment of pH.
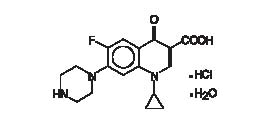
Ciprofloxacin, a fluoroquinolone is available as the monohydrochloride monohydrate salt of 1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-7-(1-piperazinyl)-3-qui nolinecarboxylic acid. The empirical formula is C17H18FN3O3·HCl·H2O and the structural formula is:
Dexamethasone,
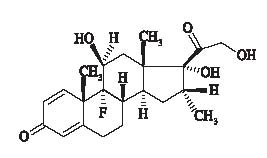
9-fluoro-11(beta),17,21-trihydroxy-16(alpha)-methylpregna-1,4-di ene-3,20-dione, is an anti-inflammatory corticosteroid. The empirical formula is C22H29FO5 and the structural formula is:
CLINICAL PHARMACOLOGY
Pharmacokinetics: Following a single bilateral 4-drop (total dose = 0.28 mL, 0.84 mg ciprofloxacin, 0.28 mg dexamethasone) topical otic dose of CIPRODEX® Otic to pediatric patients after tympanostomy tube insertion, measurable plasma concentrations of ciprofloxacin and dexamethasone were observed at 6 hours following administration in 2 of 9 patients and 5 of 9 patients, respectively.
Mean ± SD peak plasma concentrations of ciprofloxacin were 1.39 ± 0.880 ng/mL (n=9). Peak plasma concentrations ranged from 0.543 ng/mL to 3.45 ng/mL and were on average approximately 0.1% of peak plasma concentrations achieved with an oral dose of 250-mg [1]. Peak plasma concentrations of ciprofloxacin were observed within 15 minutes to 2 hours post dose application.
Mean ± SD peak plasma concentrations of dexamethasone were 1.14 ± 1.54 ng/mL (n=9). Peak plasma concentrations ranged from 0.135 ng/mL to 5.10 ng/mL and were on average approximately 14% of peak concentrations reported in the literature following an oral 0.5-mg tablet dose[2]. Peak plasma concentrations of dexamethasone were observed within 15 minutes to 2 hours post dose application.
Dexamethasone has been added to aid in the resolution of the inflammatory response accompanying bacterial infection (such as otorrhea in pediatric patients with AOM with tympanostomy tubes).
Microbiology: Ciprofloxacin has in vitro activity against a wide range of gram-positive and gram-negative microorganisms. The bactericidal action of ciprofloxacin results from interference with the enzyme, DNA gyrase, which is needed for the synthesis of bacterial DNA. Cross-resistance has been observed between ciprofloxacin and other fluoroquinolones. There is generally no cross-resistance between ciprofloxacin and other classes of antibacterial agents such as beta-lactams or aminoglycosides.
Ciprofloxacin has been shown to be active against most isolates of the following microorganisms, both in vitro and clinically in otic infections as described in the INDICATIONS AND USAGE section.
Aerobic and facultative gram-positive microorganisms
Staphylococcus aureus
Streptococcus pneumoniae
Aerobic and facultative gram-negative microorganisms
Haemophilus influenzae
Moraxella catarrhalis
Pseudomonas aeruginosa
INDICATIONS AND USAGE
CIPRODEX® Otic is indicated for the treatment of infections caused by susceptible isolates of the designated microorganisms in the specific conditions listed below:
Acute Otitis Media in pediatric patients (age 6 months and older) with tympanostomy tubes due to Staphylococcus aureus, Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Pseudomonas aeruginosa.
Acute Otitis Externa in pediatric (age 6 months and older), adult and elderly patients due to Staphylococcus aureus and Pseudomonas aeruginosa.
CONTRAINDICATIONS
CIPRODEX® Otic is contraindicated in patients with a history of hypersensitivity to ciprofloxacin, to other quinolones, or to any of the components in this medication. Use of this product is contraindicated in viral infections of the external canal including herpes simplex infections.
WARNINGS
FOR OTIC USE ONLY
(This product is not approved for ophthalmic use.)
NOT FOR INJECTION
CIPRODEX® Otic should be discontinued at the first appearance of a skin rash or any other sign of hypersensitivity. Serious and occasionally fatal hypersensitivity (anaphylactic) reactions, some following the first dose, have been reported in patients receiving systemic quinolones. Serious acute hypersensitivity reactions may require immediate emergency treatment.
PRECAUTIONS
General:
As with other antibacterial preparations, use of this product may result in overgrowth of nonsusceptible organisms, including yeast and fungi. If the infection is not improved after one week of treatment, cultures should be obtained to guide further treatment. If otorrhea persists after a full course of therapy, or if two or more episodes of otorrhea occur within six months, further evaluation is recommended to exclude an underlying condition such as cholesteatoma, foreign body, or a tumor.
The systemic administration of quinolones, including ciprofloxacin at doses much higher than given or absorbed by the otic route, has led to lesions or erosions of the cartilage in weight-bearing joints and other signs of arthropathy in immature animals of various species.
Guinea pigs dosed in the middle ear with CIPRODEX® Otic for one month exhibited no drug-related structural or functional changes of the cochlear hair cells and no lesions in the ossicles. CIPRODEX® Otic was also shown to lack dermal sensitizing potential in the guinea pig when tested according to the method of Buehler.
No signs of local irritation were found when CIPRODEX® Otic was applied topically in the rabbit eye.
Information for Patients
For otic use only. (This product is not approved for use in the eye.) Warm the bottle in your hand for one to two minutes prior to use and shake well immediately before using.
Avoid contaminating the tip with material from the ear, fingers, or other sources.
Protect from light.
If rash or allergic reaction occurs, discontinue use immediately and contact your physician.
It is very important to use the ear drops for as long as the doctor has instructed, even if the symptoms improve.
Discard unused portion after therapy is completed.
Acute Otitis Media in pediatric patients with tympanostomy tubes Prior to administration of CIPRODEX® Otic in patients (6 months and older) with acute otitis media through tympanostomy tubes, the suspension should be warmed by holding the bottle in the hand for one or two minutes to avoid dizziness which may result from the instillation of a cold suspension. The patient should lie with the affected ear upward, and then the drops should be instilled. The tragus should then be pumped 5 times by pushing inward to facilitate penetration of the drops into the middle ear. This position should be maintained for 60 seconds. Repeat, if necessary, for the opposite ear (see DOSAGE AND ADMINISTRATION).
Acute Otitis Externa
Prior to administration of CIPRODEX® Otic in patients with acute otitis externa, the suspension should be warmed by holding the bottle in the hand for one or two minutes to avoid dizziness which may result from the instillation of a cold suspension. The patient should lie with the affected ear upward, and then the drops should be instilled. This position should be maintained for 60 seconds to facilitate penetration of the drops into the ear canal. Repeat, if necessary, for the opposite ear (see DOSAGE AND ADMINISTRATION).
Drug Interactions
Specific drug interaction studies have not been conducted with CIPRODEX® Otic.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term carcinogenicity studies in mice and rats have been completed for ciprofloxacin. After daily oral doses of 750 mg/kg (mice) and 250 mg/kg (rats) were administered for up to 2 years, there was no evidence that ciprofloxacin had any carcinogenic or tumorigenic effects in these species. No long term studies of CIPRODEX® Otic have been performed to evaluate carcinogenic potential.
Eight in vitro mutagenicity tests have been conducted with ciprofloxacin, and the test results are listed below:
Salmonella/Microsome Test (Negative)
E. coli DNA Repair Assay (Negative)
Mouse Lymphoma Cell Forward Mutation
Assay (Positive)
Chinese Hamster V79 Cell HGPRT Test (Negative)
Syrian Hamster Embryo Cell Transformation
Assay (Negative)
Saccharomyces cerevisiae Point Mutation
Assay (Negative)
Saccharomyces cerevisiae Mitotic Crossover and Gene
Conversion Assay (Negative)
Rat Hepatocyte DNA Repair Assay (Positive)
Thus, 2 of the 8 tests were positive, but results of the following 3 in vivo test systems gave negative results:
Rat Hepatocyte DNA Repair Assay
Micronucleus Test (Mice)
Dominant Lethal Test (Mice)
Fertility studies performed in rats at oral doses of ciprofloxacin up to 100 mg/kg/day revealed no evidence of impairment. This would be over 100 times the maximum recommended clinical dose of ototopical ciprofloxacin based upon body surface area, assuming total absorption of ciprofloxacin from the ear of a patient treated with CIPRODEX® Otic twice per day according to label directions.
Long term studies have not been performed to evaluate the carcinogenic potential of topical otic dexamethasone. Dexamethasone has been tested for in vitro and in vivo genotoxic potential and shown to be positive in the following assays; chromosomal aberrations, sister-chromatid exchange in human lymphocytes and micronuclei and sister-chromatid exchanges in mouse bone marrow. However, the Ames/Salmonella assay, both with and without S9 mix, did not show any increase in His+ revertants.
The effect of dexamethasone on fertility has not been investigated following topical otic application. However, the lowest toxic dose of dexamethasone identified following topical dermal application was 1.802 mg/kg in a 26-week study in male rats and resulted in changes to the testes, epididymis, sperm duct, prostate, seminal vessicle, Cowper's gland and accessory glands. The relevance of this study for short term topical otic use is unknown.
Pregnancy
Teratogenic Effects. Pregnancy Category C:
Reproduction studies have been performed in rats and mice using oral doses of up to 100 mg/kg and IV doses up to 30 mg/kg and have revealed no evidence of harm to the fetus as a result of ciprofloxacin. In rabbits, ciprofloxacin (30 and 100 mg/kg orally) produced gastrointestinal disturbances resulting in maternal weight loss and an increased incidence of abortion, but no teratogenicity was observed at either dose. After intravenous administration of doses up to 20 mg/kg, no maternal toxicity was produced in the rabbit, and no embryotoxicity or teratogenicity was observed.
Corticosteroids are generally teratogenic in laboratory animals when administered systemically at relatively low dosage levels. The more potent corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
Animal reproduction studies have not been conducted with CIPRODEX® Otic. No adequate and well controlled studies have been performed in pregnant women. Caution should be exercised when CIPRODEX® Otic is used by a pregnant woman.
Nursing Mothers:
Ciprofloxacin and corticosteroids, as a class, appear in milk following oral administration. Dexamethasone in breast milk could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical otic administration of ciprofloxacin or dexamethasone could result in sufficient systemic absorption to produce detectable quantities in human milk. Because of the potential for unwanted effects in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use:
The safety and efficacy of CIPRODEX® Otic have been established in pediatric patients 6 months and older (937 patients) in adequate and well-controlled clinical trials. Although no data are available on patients less than age 6 months, there are no known safety concerns or differences in the disease process in this population that would preclude use of this product. (See DOSAGE AND ADMINISTRATION.)
No clinically relevant changes in hearing function were observed in 69 pediatric patients (age 4 to 12 years) treated with CIPRODEX® Otic and tested for audiometric parameters.
ADVERSE REACTIONS
In Phases II and III clinical trials, a total of 937 patients were treated with CIPRODEX® Otic. This included 400 patients with acute otitis media with tympanostomy tubes and 537 patients with acute otitis externa. The reported treatment-related adverse events are listed below:
Acute Otitis Media in pediatric patients with tympanostomy tubes
The following treatment-related adverse events occurred in 0.5% or more of the patients with non-intact tympanic membranes.
| Adverse Event | Incidence (N=400) |
| Ear discomfort | 3.0% |
| Ear pain | 2.3% |
| Ear precipitate (residue) | 0.5% |
| Irritability | 0.5% |
| Taste perversion | 0.5% |
The following treatment-related adverse events were each reported in a single patient: tympanostomy tube blockage; ear pruritus; tinnitus; oral moniliasis; crying; dizziness; and erythema.
Acute Otitis Externa
The following treatment-related adverse events occurred in 0.4% or more of the patients with intact tympanic membranes.
| Adverse Event | Incidence (N=537) |
| Ear pruritus | 1.5% |
| Ear debris | 0.6% |
| Superimposed ear infection | 0.6% |
| Ear congestion | 0.4% |
| Ear pain | 0.4% |
| Erythema | 0.4% |
The following treatment-related adverse events were each reported in a single patient: ear discomfort; decreased hearing; and ear disorder (tingling).
DOSAGE AND ADMINISTRATION
CIPRODEX® OTIC SHOULD BE SHAKEN WELL IMMEDIATELY BEFORE USE
CIPRODEX® Otic contains 3 mg (3000 ug/mL) ciprofloxacin and 1 mg/mL dexamethasone.
Acute Otitis Media in pediatric patients with tympanostomy tubes: The recommended dosage regimen for the treatment of acute otitis media in pediatric patients (age 6 months and older) through tympanostomy tubes is:
Four drops (0.14 mL, 0.42 mg ciprofloxacin, 0.14 mg dexamethasone) instilled into the affected ear twice daily for seven days. The suspension should be warmed by holding the bottle in the hand for one or two minutes to avoid dizziness, which may result from the instillation of a cold suspension. The patient should lie with the affected ear upward, and then the drops should be instilled. The tragus should then be pumped 5 times by pushing inward to facilitate penetration of the drops into the middle ear. This position should be maintained for 60 seconds. Repeat, if necessary, for the opposite ear. Discard unused portion after therapy is completed.
Acute Otitis Externa: The recommended dosage regimen for the treatment of acute otitis externa is: For patients (age 6 months and older): Four drops (0.14 mL, 0.42 mg ciprofloxacin, 0.14 mg dexamethasone) instilled into the affected ear twice daily for seven days. The suspension should be warmed by holding the bottle in the hand for one or two minutes to avoid dizziness, which may result from the instillation of a cold suspension. The patient should lie with the affected ear upward, and then the drops should be instilled. This position should be maintained for 60 seconds to facilitate penetration of the drops into the ear canal. Repeat, if necessary, for the opposite ear. Discard unused portion after therapy is completed.
HOW SUPPLIED
CIPRODEX® (ciprofloxacin 0.3% and dexamethasone 0.1%) Sterile Otic Suspension is supplied as follows: 7.5 mL fill in a DROP-TAINER® system. The DROP-TAINER® system consists
of a natural polyethylene bottle and natural plug, with a white polypropylene closure. Tamper evidence is provided with a shrink band around the closure and neck area of the package.
NDC 21695-969-75, 7.5 mL fill
Storage:
Store at controlled room temperature, 15°C to 30°C (59°F to 86°F). Avoid freezing. Protect from light.
Clinical Studies:
In a randomized, multicenter, controlled clinical trial, CIPRODEX® Otic dosed 2 times per day for 7 days demonstrated clinical cures in the per protocol analysis in 86% of AOMT patients compared to 79% for ofloxacin solution, 0.3%, dosed 2 times per day for 10 days. Among culture positive patients, clinical cures were 90% for CIPRODEX® Otic compared to 79% for ofloxacin solution, 0.3%. Microbiological eradication rates for these patients in the same clinical trial were 91% for CIPRODEX® Otic compared to 82% for ofloxacin solution, 0.3%. In 2 randomized multicenter, controlled clinical trials, CIPRODEX® Otic dosed 2 times per day for 7 days demonstrated clinical cures in 87% and 94% of per protocol evaluable AOE patients, respectively, compared to 84% and 89%, respectively, for otic suspension containing neomycin 0.35%, polymyxin B 10,000 IU/mL, and hydrocortisone 1.0% (neo/poly/HC). Among culture positive patients clinical cures were 86% and 92% for CIPRODEX® Otic compared to 84% and 89%, respectively, for neo/poly/HC. Microbiological eradication rates for these patients in the same clinical trials were 86% and 92% for CIPRODEX® Otic compared to 85% and 85%, respectively, for neo/poly/HC.
References:
1. Campoli-Richards DM, Monk JP, Price A, Benfield P, Todd PA, Ward A. Ciprofloxacin: A review of its antibacterial activity, pharmacokinetic properties and therapeutic use. Drugs 1988;35:373-447.
2. Loew D, Schuster O, and Graul E. Dose-dependent pharmacokinetics of dexamethasone. Eur J Clin Pharmacol 1986;30:225-230.
U.S. Patent Nos. 6,284,804; 6,359,016
Licensed to Alcon, Inc. by Bayer Schering Pharma AG.
CIPRODEX is a registered trademark of Bayer AG, licensed to Alcon, Inc by Bayer AG.
Rx Only
©2003, 2004, 2008, 2009 Alcon, Inc.
Revision date: 17 July 2003
Relabeled by: Rebel Distributors Corp
Thousand Oaks, CA 91320
PATIENT INFORMATION
CIPRODEX® (CI-PRO-DEX)
(ciprofloxacin 0.3% and dexamethasone 0.1%)
Sterile Otic Suspension
IMPORTANT PATIENT INFORMATION AND INSTRUCTIONS. READ BEFORE USE.
What is CIPRODEX® Otic?
CIPRODEX® Otic is an antibiotic/steroid combination product in a sterile suspension used to treat:
• Middle Ear Infection with Drainage Through a Tube in Children 6 months and older: A middle ear infection is a bacterial infection behind the eardrum. People with a tube in the eardrum may notice drainage from the ear canal.\
• Outer Ear Canal Infection in Patients 6 months and older: An outer ear canal infection, also known as "Swimmer's Ear", is a bacterial infection of the outer ear canal. The ear canal and the outer part of the ear may swell, turn red, and be painful. Also, a fluid discharge may appear in the ear canal.
Who should NOT use CIPRODEX® Otic?
• Do not use this product if allergic to ciprofloxacin or to other quinolone antibiotics.
• Do not use this product if allergic to dexamethasone or to other steroids.
• Do not give this product to pediatric patients who are less than 6 months old.
How often should CIPRODEX® Otic be given?
CIPRODEX® Otic ear drops should be given 2 times each day (about 12 hours apart, for example, 8 AM and 8 PM) in each infected ear unless the doctor has instructed otherwise. The best times to use the ear drops are in the morning and at night. It is very important to use the ear drops for as long as the doctor has instructed, even if the symptoms improve. If CIPRODEX® Otic ear drops are not used for as long as the doctor has instructed, the infection may return.
What if a dose is missed?
If a dose of CIPRODEX® Otic is missed, it should be given as soon as possible. If it is almost time for the next dose, skip the missed dose and go back to the regular dosing schedule. Do not use a double dose unless the doctor has instructed you to do so. If the infection is not improved after one week, you should consult your doctor. If you have two or more episodes of drainage within six months, it is recommended you see your doctor for further evaluation.
What activities should be avoided while using CIPRODEX® Otic?
It is important that the infected ear(s) remain clean and dry. When bathing, avoid getting the infected ear(s) wet. Avoid swimming unless the doctor has instructed otherwise.
What are the possible side effects of CIPRODEX® Otic?
During the testing of CIPRODEX® Otic for middle ear infections, the most common side effect related to CIPRODEX® Otic was ear discomfort that occurred in up to 3 out of 100 patients. Other common side effects were: ear pain; ear precipitate (residue); irritability; and abnormal taste. During the testing of CIPRODEX® Otic for ear canal infections, the most common side effect related to CIPRODEX® Otic was itching of the ear that occurred in 1 to 2 out of 100 patients. Other common side effects were: ear debris; ear infection in the treated ear; ear congestion; ear pain; and rash.
If any of these side effects persist, call the doctor.
If an allergic reaction to CIPRODEX® Otic occurs, stop using the product and contact your doctor.
DO NOT TAKE BY MOUTH
If CIPRODEX® Otic is accidentally swallowed or overdose occurs, call the doctor immediately. This medicine is available only with a doctor's prescription. Use only as directed. Do not use this medicine if outdated. If you wish to learn more about CIPRODEX® Otic, call your doctor or pharmacist.
HOW SUPPLIED
CIPRODEX® Otic is supplied as follows: 7.5 mL fill in a DROP-TAINER® system. The DROP-TAINER® system consists of a natural polyethylene bottle and natural plug, with a white polypropylene closure. Tamper evidence is provided with a shrink band around the closure and neck area of the package.
NDC 21695-969-75, 7.5 mL fill
Storage:
Store at controlled room temperature, 15°C to 30°C (59°F to 86°F). Avoid freezing. Protect from light.
U.S. Patent Nos. 6,284,804; 6,359,016
Licensed to Alcon, Inc. by Bayer Schering Pharma AG.
CIPRODEX is a registered trademark of Bayer AG, licensed to Alcon, Inc by Bayer AG.
Rx Only
©2003, 2004, 2008, 2009 Alcon, Inc.
Relabeled by: Rebel Distributors Corp
Thousand Oaks, CA 91320
How should CIPRODEX® Otic be given?
1. Wash hands
The person giving CIPRODEX® Otic should wash his/her hands with soap and water.
2. Warm & shake bottle
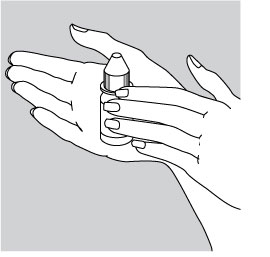
Hold the bottle of CIPRODEX® Otic in the hand for one or two minutes to warm the suspension, then shake well.
3. Add drops
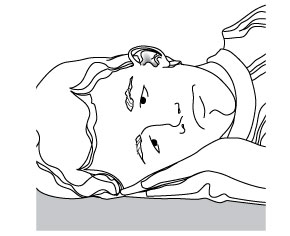
The person receiving CIPRODEX® Otic should lie on his/her side with the infected ear up.
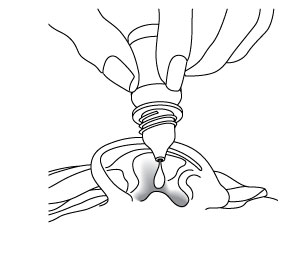
Patients should have 4 drops of CIPRODEX® Otic put into the infected ear. The tip of the bottle should not touch the fingers, or the ear, or any other surfaces.
BE SURE TO FOLLOW INSTRUCTIONS BELOW FOR THE PATIENT'S SPECIFIC EAR INFECTION.
4. For Patients with Middle Ear Infection with Tubes:
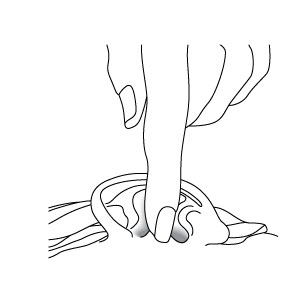
While the person receiving CIPRODEX® Otic lies on his/her side, the person giving the drops should gently press the tragus (see diagram) 5 times in a pumping motion. This will allow the drops to pass through the tube in the eardrum and into the middle ear.
5. For Patients with Outer Ear Infection ("Swimmer's Ear"):
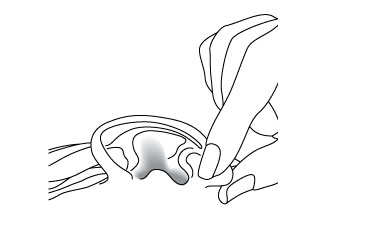
While the person receiving the drops lies on his/her side, the person giving the drops should gently pull the outer ear lobe upward and backward. This will allow the ear drops to flow down into the ear canal.
6. Stay on side
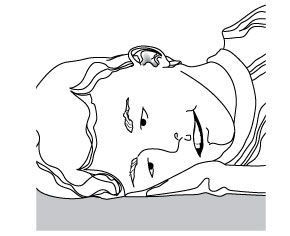
The person who received the ear drops should remain on his/her side for at least 60 seconds.
Repeat Steps 2-5 for the other ear if both ears are infected.
345579-0709