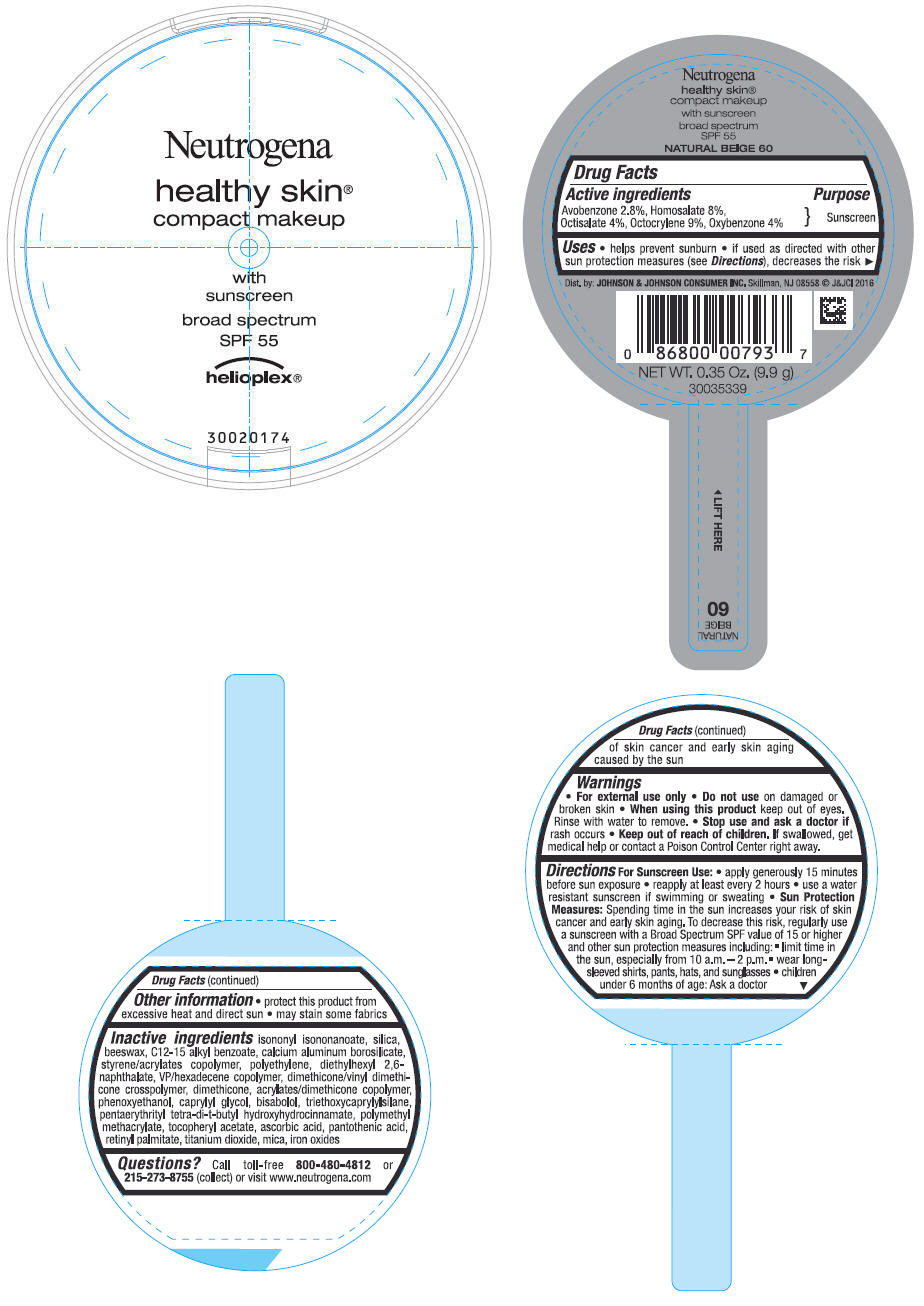
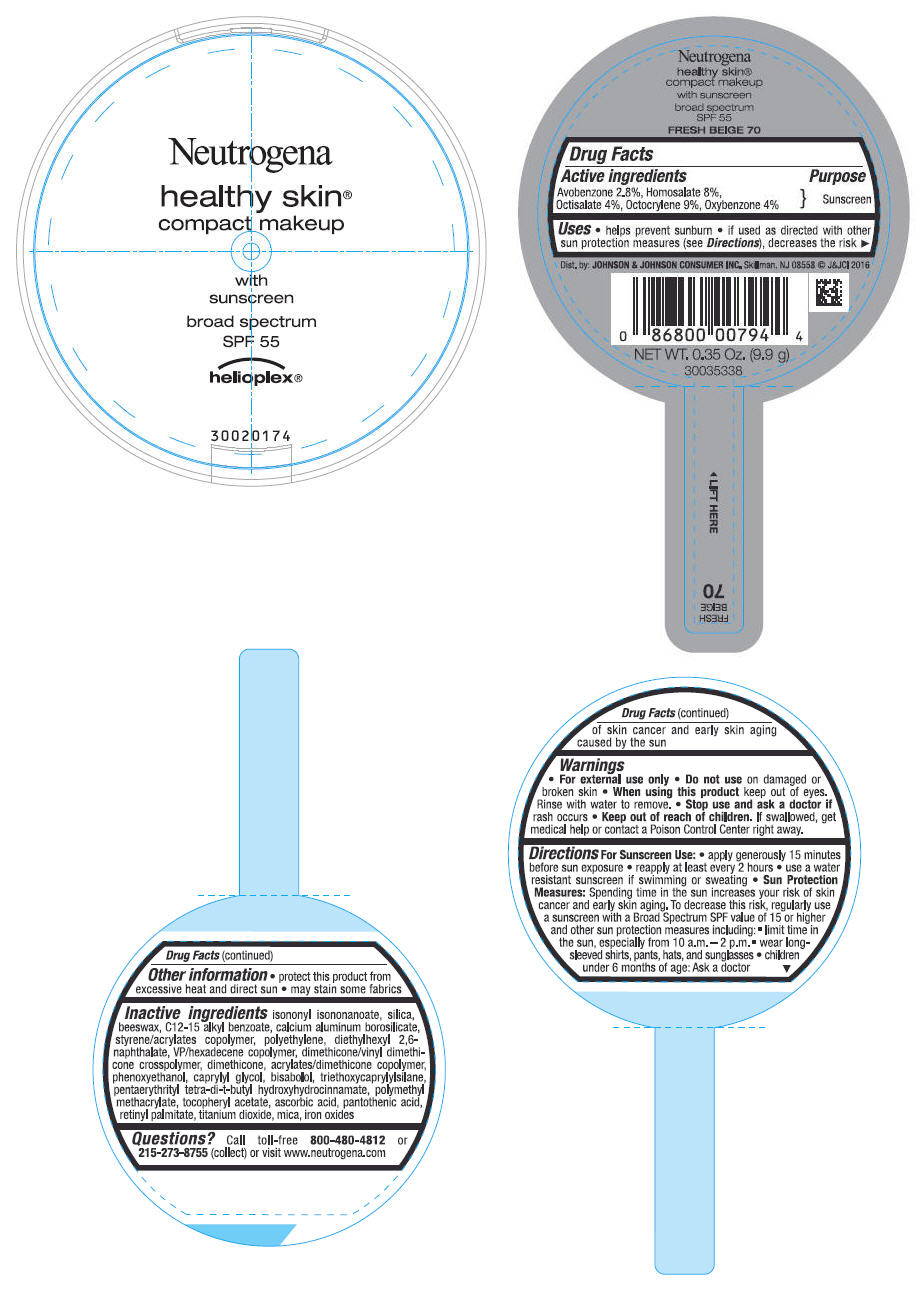
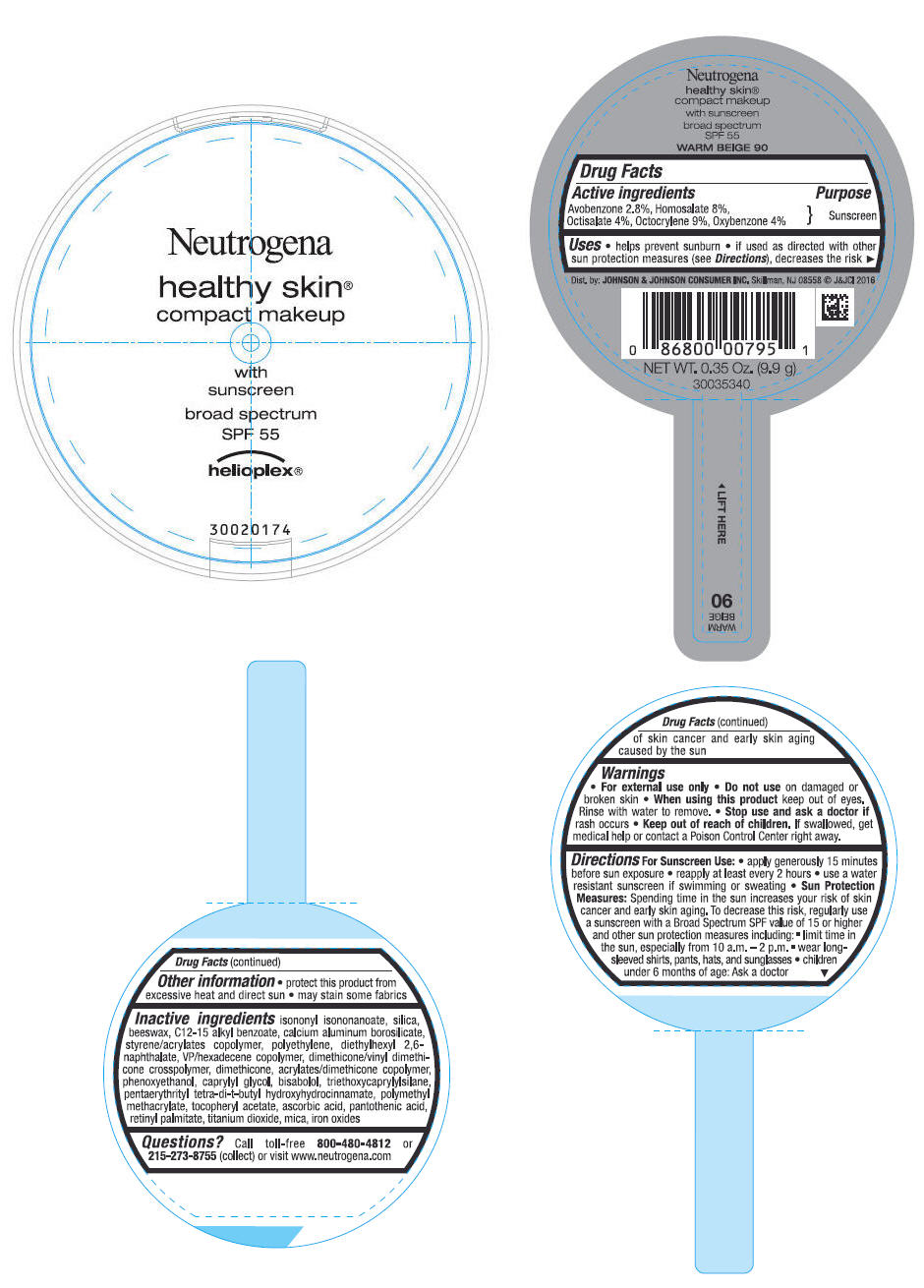
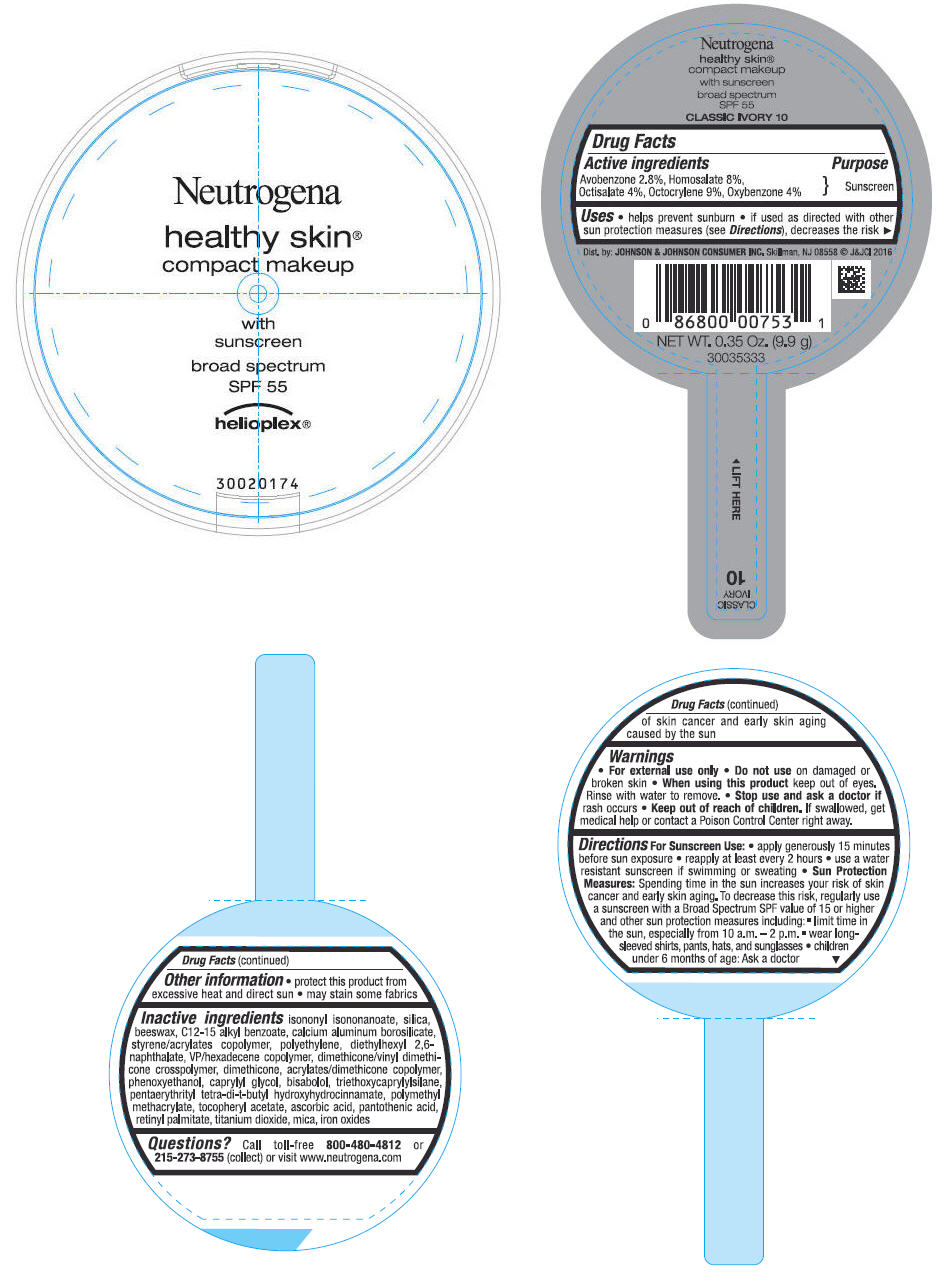
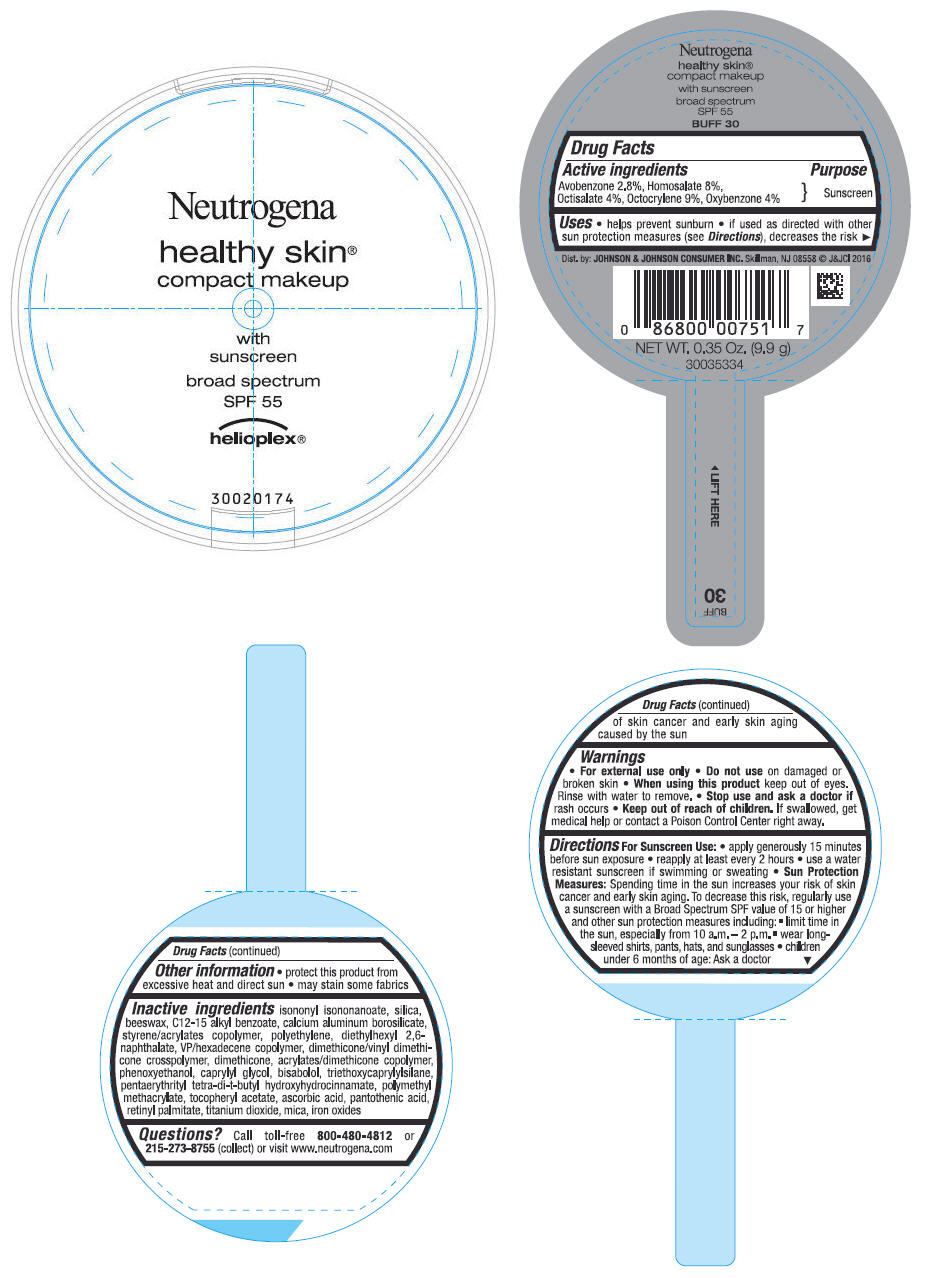
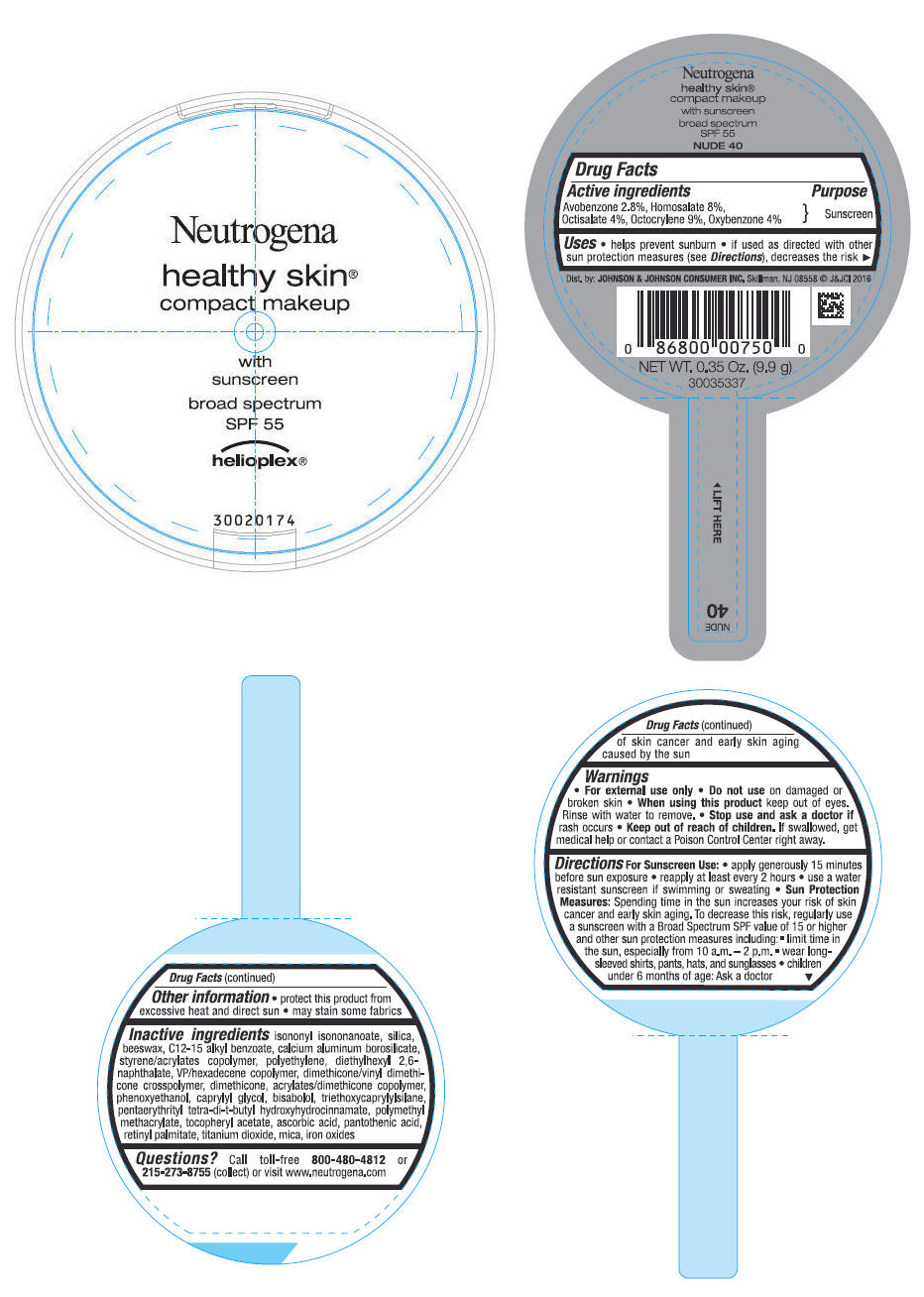
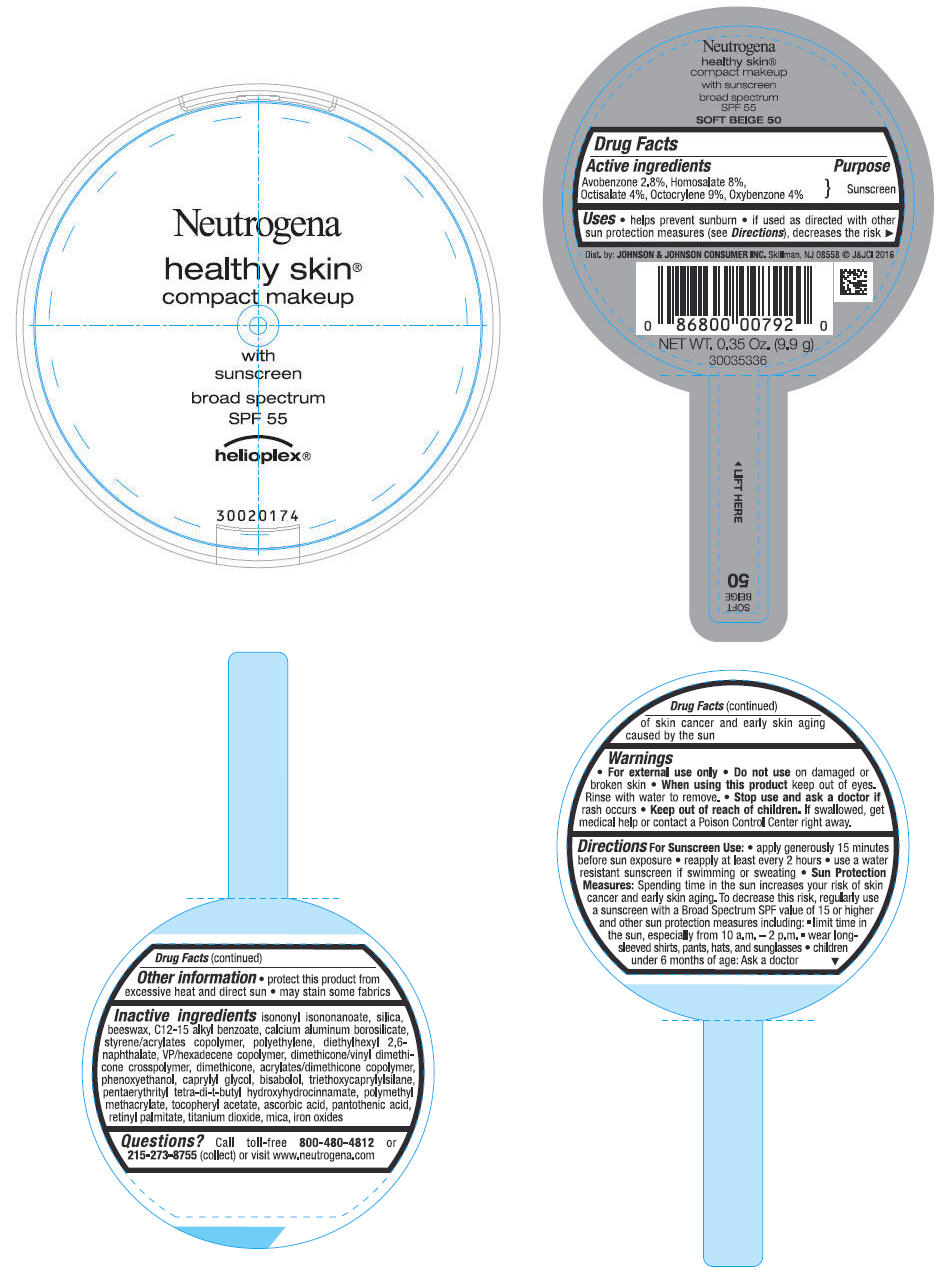
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions), decreases the risk of skin cancer and early skin aging caused by the sun
Directions
For Sunscreen Use:
- apply generously 15 minutes before sun exposure
- reapply at least every 2 hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures: Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: Ask a doctor
Inactive ingredients
isononyl isononanoate, silica, beeswax, C12-15 alkyl benzoate, calcium aluminum borosilicate, styrene/acrylates copolymer, polyethylene, diethylhexyl 2,6-naphthalate, VP/hexadecene copolymer, dimethicone/vinyl dimethicone crosspolymer, dimethicone, acrylates/dimethicone copolymer, phenoxyethanol, caprylyl glycol, bisabolol, triethoxycaprylylsilane, pentaerythrityl tetra-di-t-butyl hydroxyhydrocinnamate, polymethyl methacrylate, tocopheryl acetate, ascorbic acid, pantothenic acid, retinyl palmitate, titanium dioxide, mica, iron oxides
PRINCIPAL DISPLAY PANEL - 9.9 g Container Label - CLASSIC IVORY 10
Neutrogena
healthy skin
®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex ®
PRINCIPAL DISPLAY PANEL - 9.9 g Container Label - NATURAL IVORY 20
Neutrogena
healthy skin
®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex ®

PRINCIPAL DISPLAY PANEL - 9.9 g Container Label - BUFF 30
Neutrogena
healthy skin
®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex ®
PRINCIPAL DISPLAY PANEL - 9.9 g Container Label - NUDE 40
Neutrogena
healthy skin
®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex ®
PRINCIPAL DISPLAY PANEL - 9.9 g Container Label - SOFT BEIGE 50
Neutrogena
healthy skin
®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex ®
PRINCIPAL DISPLAY PANEL - 9.9 g Container Label - NATURAL BEIGE 60
Neutrogena
healthy skin
®
compact makeup
with
sunscreen
broad spectrum
SPF 55
helioplex ®