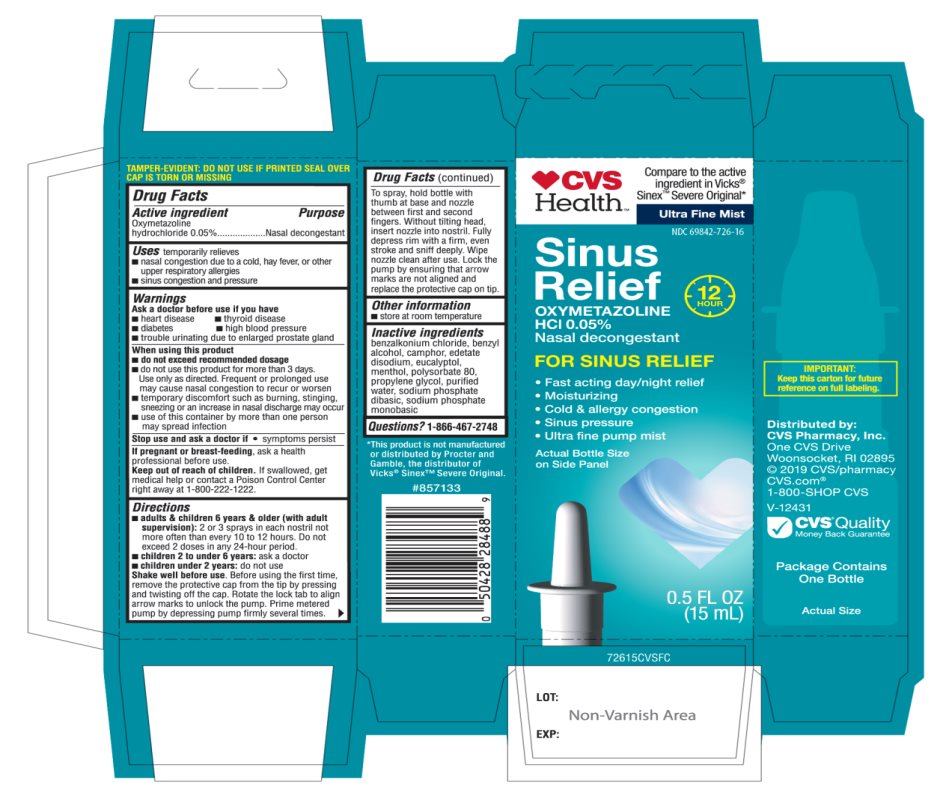
Uses
temporarily relieves
- ▪
- nasal congestion due to a cold, heavy fever, or other upper respiratory allergies
- ▪
- Sinus congestion and pressure
Ask a doctor before use if you have
- ▪
- heart disease
- ▪
- high blood pressure
- ▪
- diabetes
- ▪
- thyroid disease
- ▪
- trouble urinating due to an enlarged prostate gland
When using this product
- ▪
- do not exceed recommended dosage
- ▪
- do not use this product for more than 3 days. Use only as directed. Frequent or prolonged use may cause nasal congestion to recur or worsen.
- ▪
- temporary discomfort such as burning, stinging, sneezing or an increase in nasal discharge may occur
- ▪
- use of this container by more than one person may spread infection
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away at (1-800-222-1222).
Directions
- ▪
- adults & children 6 years & older (with adult supervision): 2 or 3 sprays in each nostril not more often than every 10 to 12 hours. Do not exceed 2 doses in any 24-hour period.
- ▪
- Children 2 to under 6 years: ask a doctor
- ▪
- Children 2 years: do not use
To Use: Shake well before use. Push down cap while turning counter-clockwise and remove cap. Remove clip under rim. Before using the first time, prime metered pump by depressing pump firmly several times. To spray, hold bottle with thumb at base and nozzle between first and second fingers without titling head, insert nozzle into nostril. Fully depress rim with a firm, even stroke and sniff deeply. Wipe nozzle clean after use. Replace clip under rim and secure cap after use.
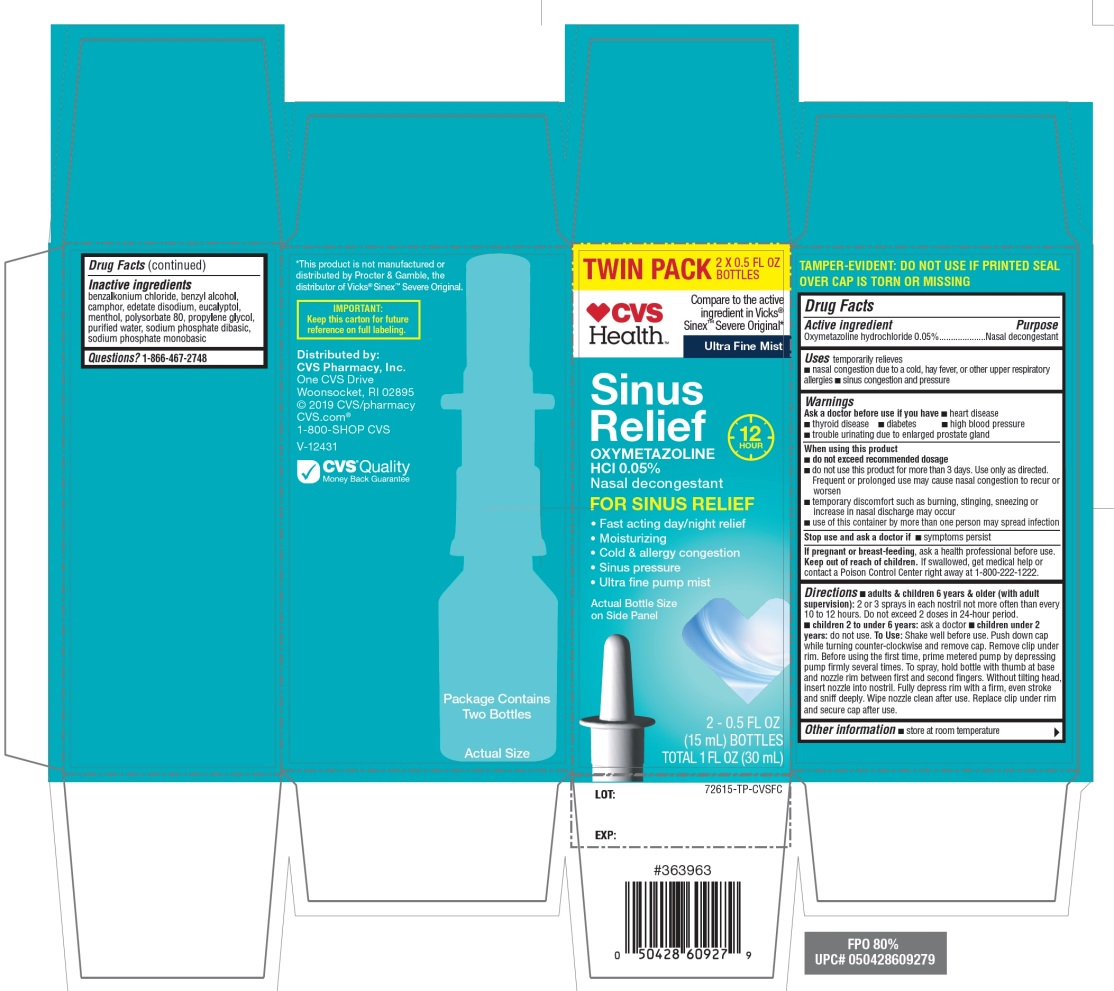
Inactive ingredients
benzalkonium chloride, benzyl alcohol, camphor, edetate disodium, eucalyptol, menthol, polysorbate 80, propylene glycol, purified water, sodium phosphate dibasic, sodium phosphate monobasic.
Principal Display Panel
CVS Health™
Compare to the active ingredient in Vicks® Sinex™ Severe Original*
Ultra-Fine Mist
12 HOUR
Sinus Relief
OXYMETAZOLINE HCl 0.05%
Nasal decongestant
FOR SINUS RELIEF
- •
- Fast acting day/night relief
- •
- Moisturizing
- •
- Cold & allergy congestion
- •
- Sinus pressure
- •
- Ultra fine pump mist
Actual Bottle Size on Side Panel
|
IMPORTANT: KEEP THE CARTON FOR FUTURE REFERENCE ON FULL LABELING |
Distributed by:
CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
2019 CVS/pharmacy
CVS.com
1-800-SHOP CVS, V-12431
- ✓
- CVS Quality
Money Back Guarantee
Package contains One Bottle B
*This product is not manufactured or distributed by Procter and Gamble, the distributer of Vicks® Sinex™ Severe Original.
PACKAGE LABEL 15 mL