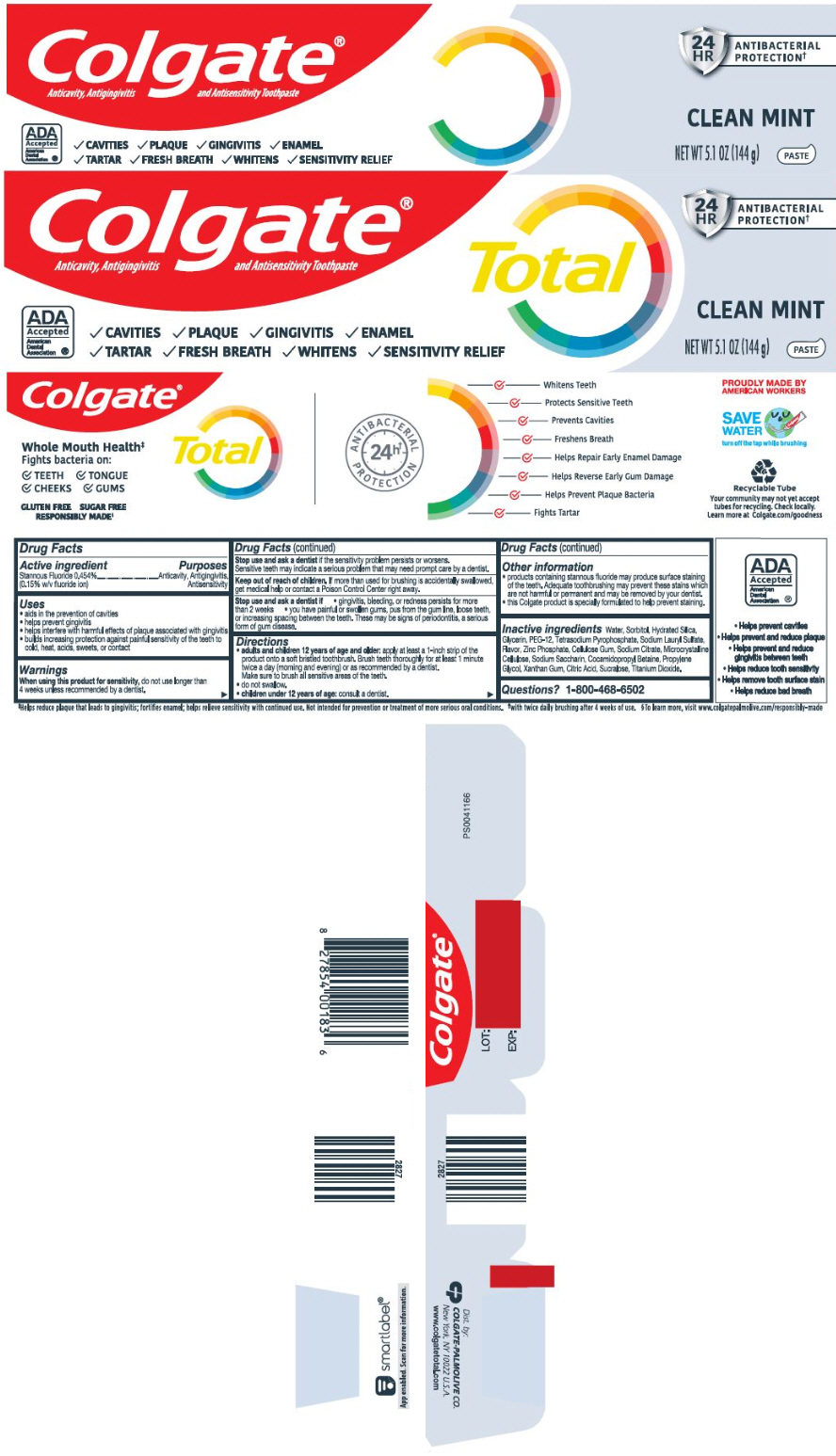
Uses
- aids in the prevention of cavities
- helps prevent gingivitis
- helps interfere with harmful effects of plaque associated with gingivitis
- builds increasing protection against painful sensitivity of the teeth to cold, heat, acids, sweets, or contact
Warnings
When using this product for sensitivity, do not use longer than 4 weeks unless recommended by a dentist.
Stop use and ask a dentist if the sensitivity problem persists or worsens. Sensitive teeth may indicate a serious problem that may need prompt care by a dentist.
Directions
-
adults and children 12 years of age and older: apply at least a 1-inch strip of the product onto a soft bristled toothbrush. Brush teeth thoroughly for at least 1 minute twice a day (morning and evening) or as recommended by a dentist.
Make sure to brush all sensitive areas of the teeth. - do not swallow.
- children under 12 years of age: consult a dentist.
Other information
- products containing stannous fluoride may produce surface staining of the teeth. Adequate toothbrushing may prevent these stains which are not harmful or permanent and may be removed by your dentist.
- this Colgate product is specially formulated to help prevent staining.
Inactive ingredients
water, sorbitol, hydrated silica, glycerin, PEG-12, tetrasodium pyrophosphate, sodium lauryl sulfate, microcrystalline cellulose, sodium citrate, zinc phosphate, flavor, cellulose gum, sodium saccharin, cocamidopropyl betaine, propylene glycol, xanthan gum, citric acid, sucralose, titanium dioxide
PRINCIPAL DISPLAY PANEL - 144 g Tube Carton
Colgate®
Anticavity, Antigingivitis and Antisensitivity Toothpaste
ADA
Accepted
American
Dental
Association ®
✓CAVITIES ✓PLAQUE ✓GINGIVITIS ✓ENAMEL
✓TARTAR ✓FRESH BREATH ✓WHITENS ✓SENSITIVITY RELIEF
Total
24
HR
ANTIBACTERIAL
PROTECTION†
CLEAN MINT
NET WT 5.1 OZ (144 g)
PASTE