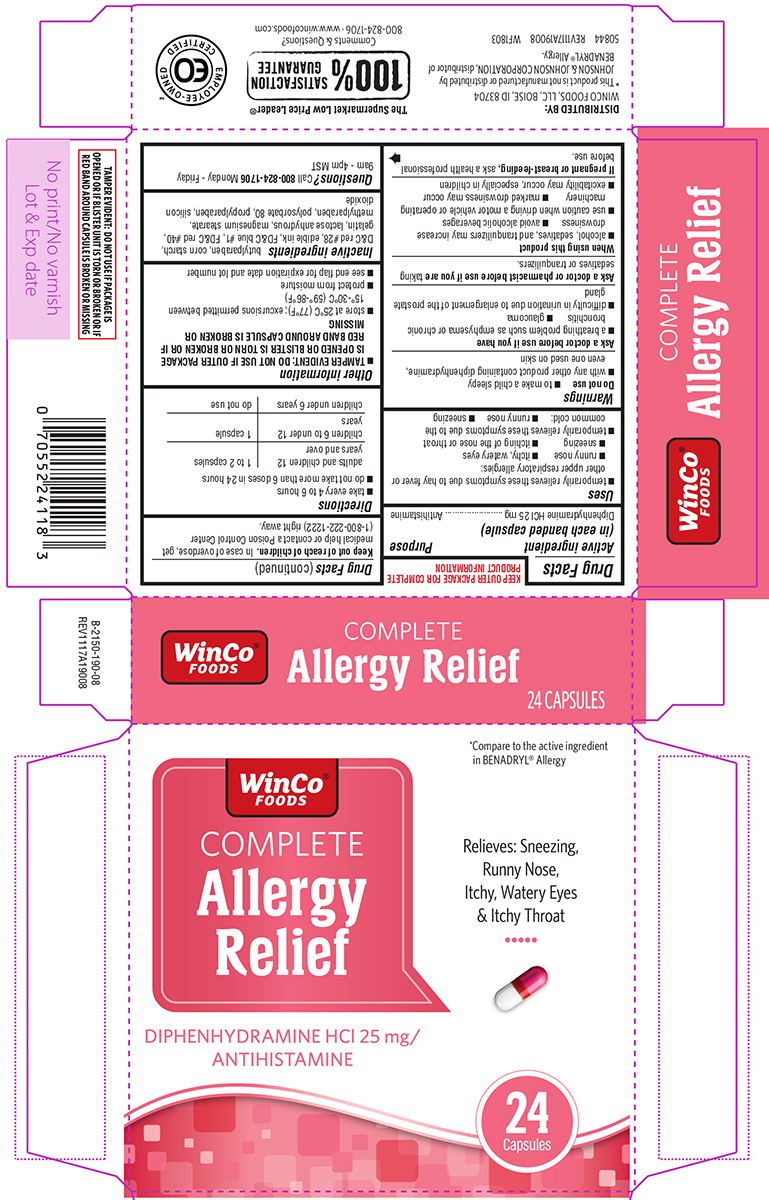
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
- temporarily relieves these symptoms due to the common cold:
- runny nose
- sneezing
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- a breathing problem such as emphysema or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
Directions
- take every 4 to 6 hours
- do not take more than 6 doses in 24 hours
| adults and children 12 years and over | 1 to 2 capsules |
| children 6 to under 12 years | 1 capsule |
| children under 6 years | do not use |
Other information
- TAMPER EVIDENT: DO NOT USE IF OUTER PACKAGE IS OPENED OR BLISTER IS TORN OR BROKEN OR IF RED BAND AROUND CAPSULE IS BROKEN OR MISSING
- store at 25ºC (77ºF); excursions permitted between 15º-30ºC (59º-86ºF)
- protect from moisture
- see end flap for expiration date and lot number
Inactive ingredients
butylparaben, corn starch, D&C red #28, edible ink, FD&C blue #1, FD&C red #40, gelatin, lactose anhydrous, magnesium stearate, methylparaben, polysorbate 80, propylparaben, silicon dioxide
Principal Display Panel
WinCo™
FOODS
COMPLETE
Allergy
Relief
DIPHENHYDRAMINE HCl 25 mg/
ANTIHISTAMINE
*Compare to the active ingredient
in BENADRYL® Allergy
Relieves: Sneezing,
Runny Nose,
Itchy, Watery Eyes
& Itchy Throat
24
Capsules
TAMPER EVIDENT: DO NOT USE IF PACKAGE IS
OPENED OR IF BLISTER UNIT IS TORN OR BROKEN OR IF
RED BAND AROUND CAPSULE IS BROKEN OR MISSING
DISTRIBUTED BY:
WINCO FOODS, LLC, BOISE, ID 83704
*This product is not manufactured or distributed by
JOHNSON & JOHNSON CORPORATION, distributor of
BENADRYL® Allergy.
50844 REV1117A19008 WF1803
100% SATISFACTION GUARANTEE
Comments & Questions?
800-824-1706 • www.wincofoods.com
Winco 44-190