INDICATIONS:
For staining the anterior segment of the eye when fitting contact lenses, in disclosing corneal injury and in applanation tonometry.
DIRECTIONS FOR USE:
To ensure full fluorescence and patient comfort, the Ful-Glo impregnated tip should be moistened before application. One or two drops of sterile irrigation solution should be used for this purpose. Touch conjunctiva or fornix as required with moistened tip. It is recommended that the patient blink several times after application.
NOTE: Contents may not be sterile if individual strip package has been damaged or previously opened. Store below 30°C.
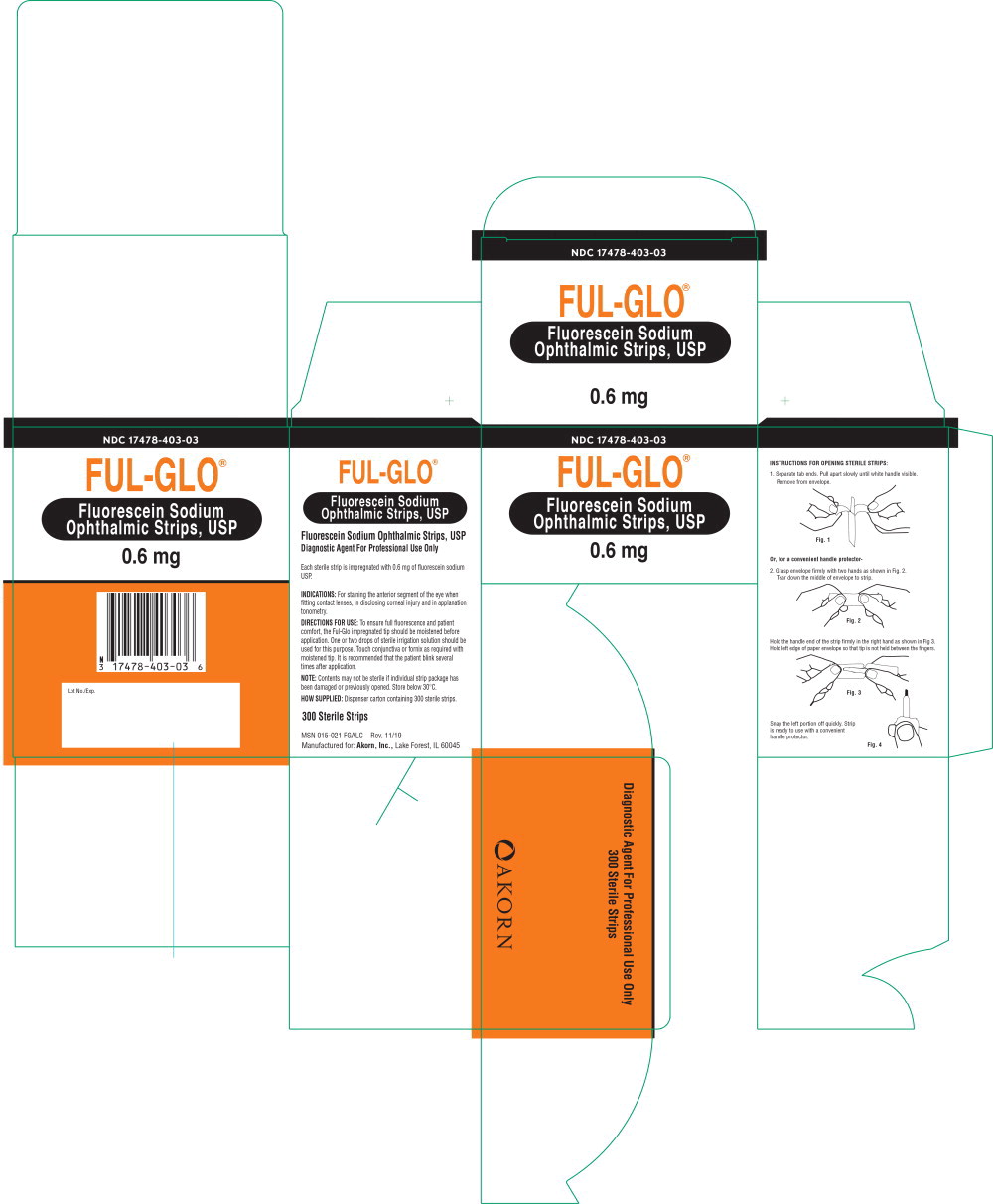
INSTRUCTIONS FOR OPENING STERILE STRIPS:
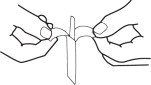
1. Separate tab ends. Pull apart slowly until white handle visible. Remove from envelope.
Or, for a convenient handle protector-
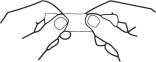
2. Grasp envelope firmly with two hands as shown in Fig. 2. Tear down the middle of envelope to strip.
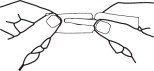
Hold the handle end of the strip firmly in the right hand as shown in Fig 3. Hold left edge of paper envelope so that tip is not held between the fingers.
Snap the left portion off quickly. Strip is ready to use with a convenient handle protector.