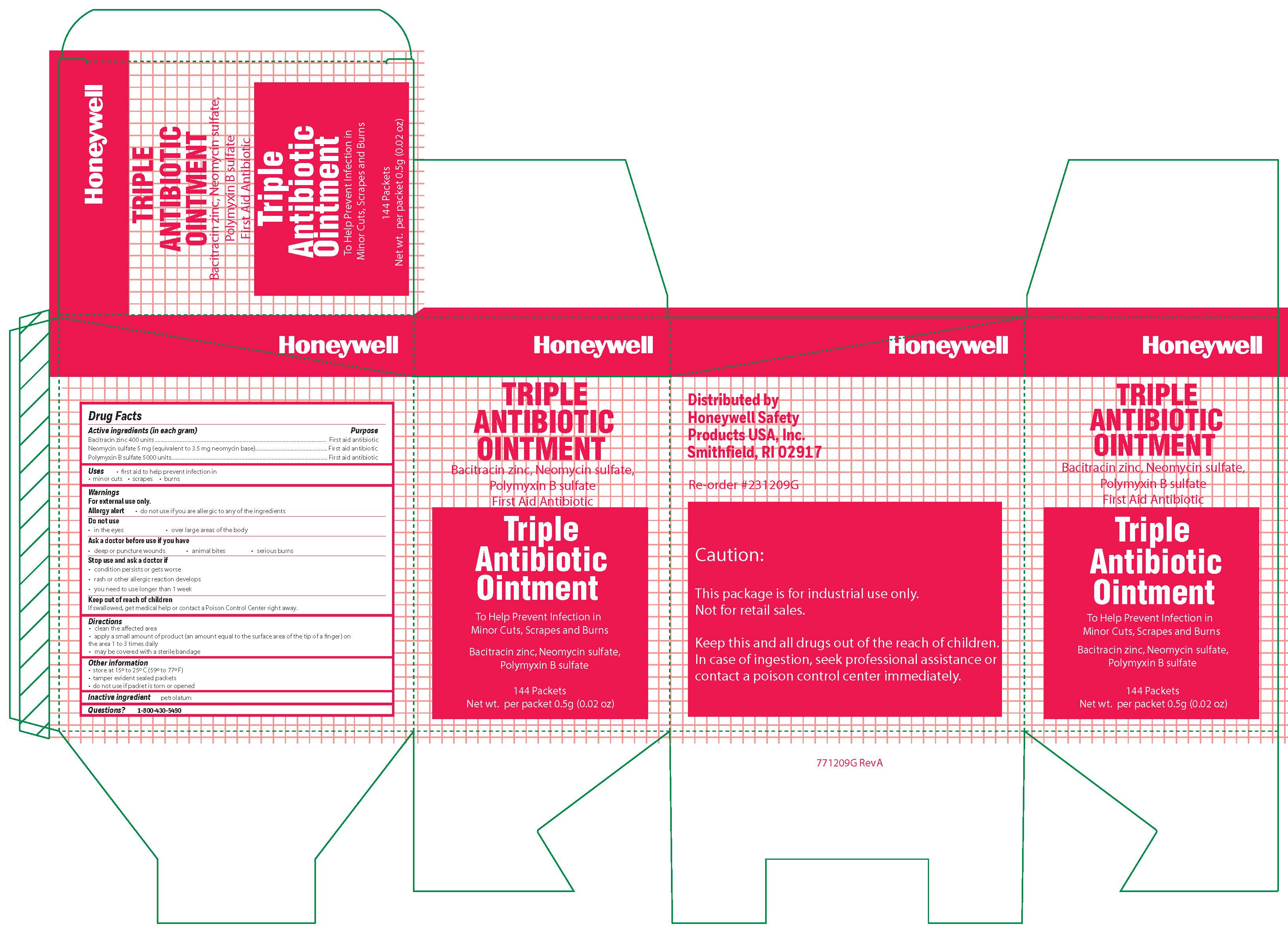
Triple
Active ingredient
Bacitracin zinc 400 units
Neomycin sulfate (5 mg equivalent to 3.5 mg Neomycin base)
Polymyxin B sulfate 5000 units
Triple
Warnings
For external use only
Allergy alert: do not use if you are allergic to any of the ingredients
Do not use
- in the eyes
- over large areas of the body
- Ask a doctor before use if you have
- a deep or puncture wounds
- animal bites
- serious burns
Triple
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Triple
Other information
- store at 15 0 to 25 0 C (59 0 to 77 0 F)
- tamper evident sealed packets
- do not use if packet is torn or opened
BZK Wipe
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
BZK Wipe
Other information
- store at room temperature 15 0 to 30 0 C (59 0 - 86 0 F)
- do not reuse towelette
Eyewash
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyewash
Warnings
For external use only
- Obtain immediate medical treatment for all open wounds in or near eyes.
- To avoid contamination, do not touch tip of container to any surface.
- Do not reuse.
- Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Eyewash
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
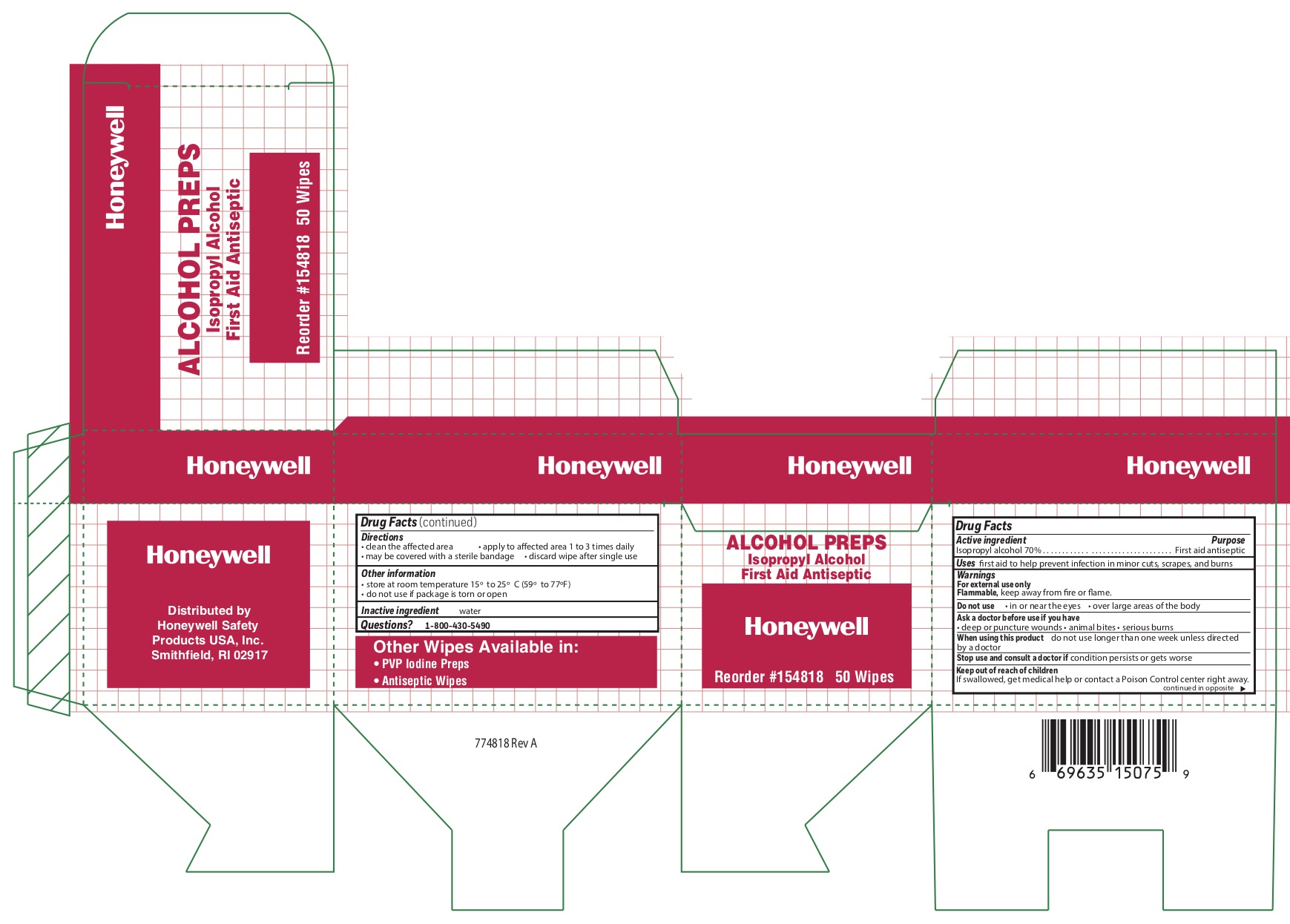
Alcohol
Directions
- clean the affected area
- may be covered with a sterile bandage
- apply wipe to affeted are 1 to 3 times daily
- discard wipe after single use
Alcohol
Other information
- store at room temperature 15 0 to 25 0 C (59 0 to 77 0 F)
- do not use if packet is torn or opened
4317
SF00004535 Kit Contents
1 TWEEZER PLASTICS 4"
1 GAUZE CLEAN-WRAP BDGE N/S 2"
2 ABD COMBINE PAD 5" X 9"
1 MICROSHIELD BAGGED 72-151
1 1 OZ, BUFF EYEWASH
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
6 BZK ANTISEPTIC WIPE, BULK
4 PR LRG NITRILE GLVES ZIP BAG
2 TRIPLE BIOTIC FOIL PACK EACH
1 TAPE ADHESIVE 1/2 X 2.5 125133
2 1/8 BURN JEL POUCH
2 ADH BNDG PLASTIC EX-LG 4"X 2"
5 ADH BANDAGE BUTTERFLY 1980000
10 WIPE ALCOHOL PREP IPA 70% (DUKAL)
1 KIT STL 10 UN WHITE 01
1 COLD PACK UNIT 4"X6" BULK
4 GAUZE PADS 2"X2" 12PLY
2 PLASTIC BANDAGE 3/4" X 3"
2 WOVEN FINGERTIP BANDAGE 3"
2 WOVEN KNUCKLE BANDAGE