4129 FIRST AID KIT- 4129 first aid kit
Honeywell Safety Products USA, INC
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
0498-4129: First Aid Kit (alcohol wipes, HC cr, BZK wipe, Pyrocaine Sp, PVP wipe, amm. Inh, Sting relief- SF00004226)
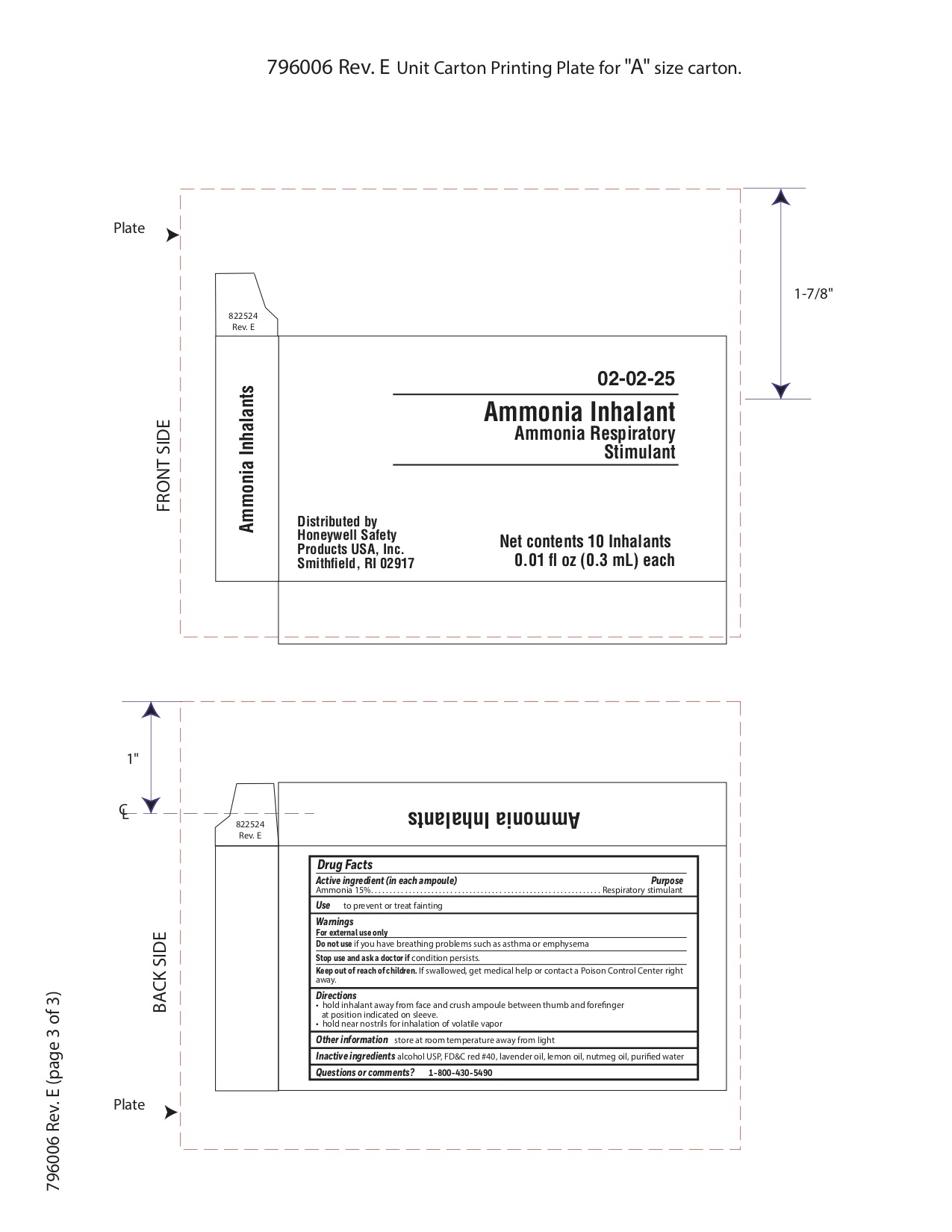
Ammonia
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Ammonia
Inactive ingredient
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
Pyrocaine
Uses
For the temporary relief of pain and itching, and to help protect against skin infection in:
- minor burns
- minor skin irritations
- minor cuts and scrapes
- insect bites
- sunburns
Pyrocaine
Warnings
For external use only
Flammable
- keep away from fire or flame
- contents under pressure
- do not puncture, incinerate or expose container to temperatures above 120 o F
Pyrocaine
Directions
- clean the affected area
- shake can well before using
- hold can 6 to 12 inches away from the affected area and spray liberally
- apply to affected area not more than 3 times daily
- for adult institutional use only
- not intended for use on children
Pyrocaine
Other information
- avoid inhaling
- use only as directed
- intentional misuse by deliberately concentrating and inhaling the contents may be harmful or fatal
PVP
Warnings
For external use only.
PVP
Directions
- clean the affected area
- apply1 to 3 times daily
- may be covered with a sterile bandage
- if bandaged, let dry first
- discard wipe after single use
PVP
Other information
- do not use on individuals who are allergic or sensitive to iodine
- store at controlled temperature 59-86ºF (15-30ºC)
- do not use if pouch is open or torn
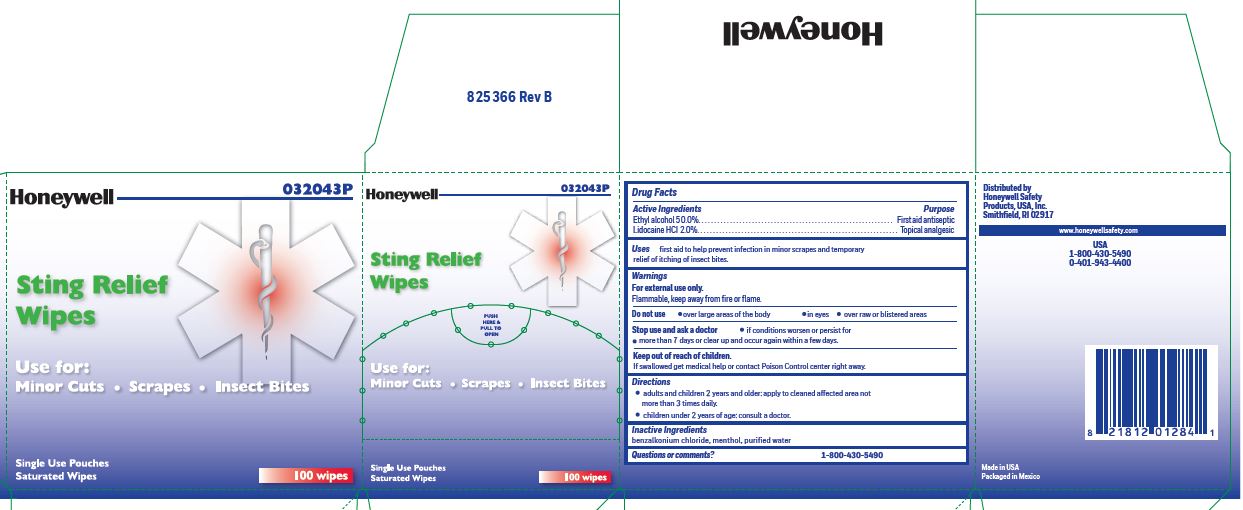
Sting Relef
Uses
- prevent infection in minor scrapes, and temporary relief of itching of insect bites
Sting Relief
Warnings
For external use only
Flammable, keep away from open fire or flame
Sting Relief
Directions
- adults and children 2 years and older: Apply to cleaned affected area not more than 3 times daily.
- children under 2 years of age: consult a doctor.
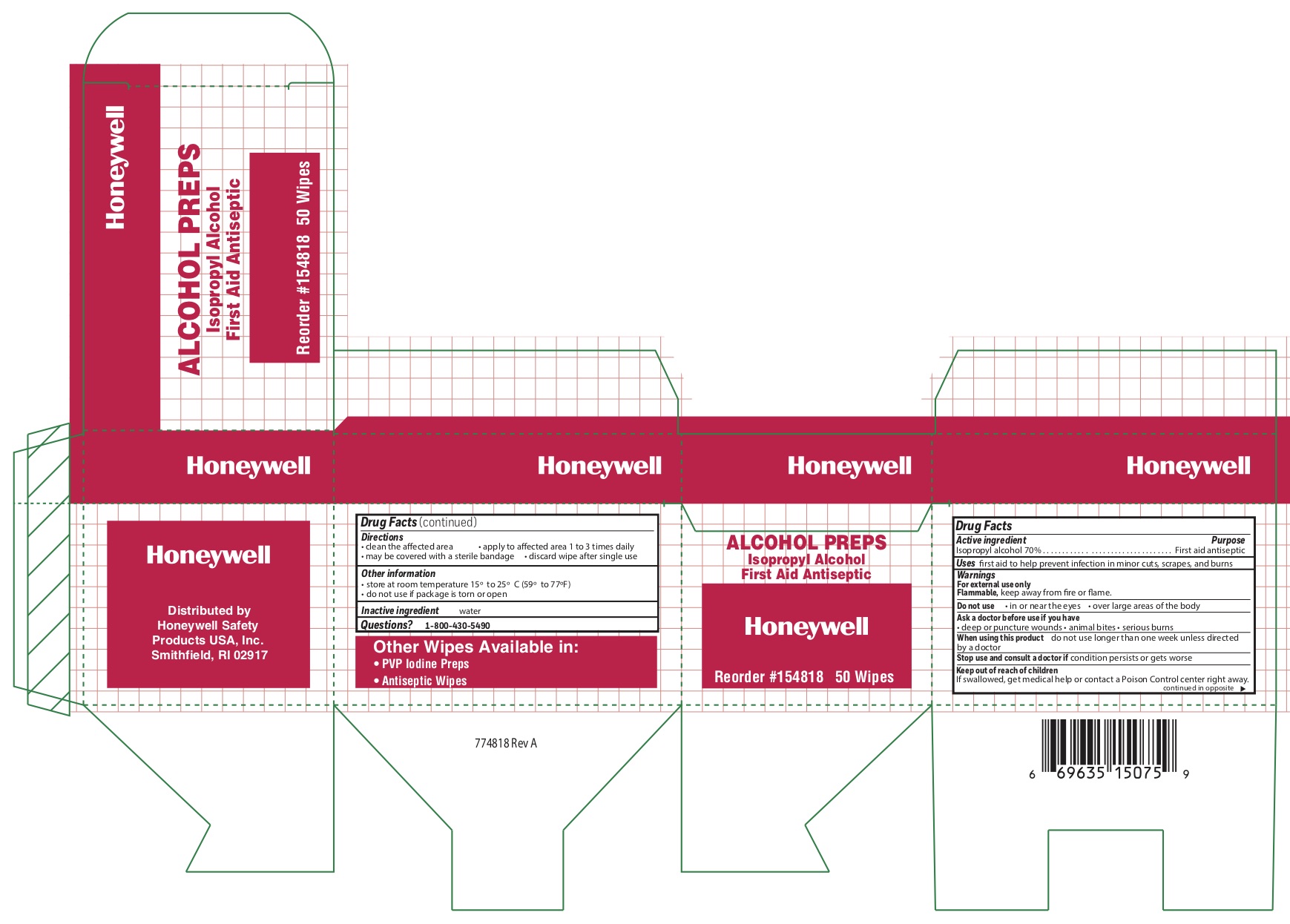
Alcohol
Directions
- clean the affected area
- may be covered with a sterile bandage
- apply wipe to affeted are 1 to 3 times daily
- discard wipe after single use
Alcohol
Other information
- store at room temperature 15 0 to 25 0 C (59 0 to 77 0 F)
- do not use if packet is torn or opened
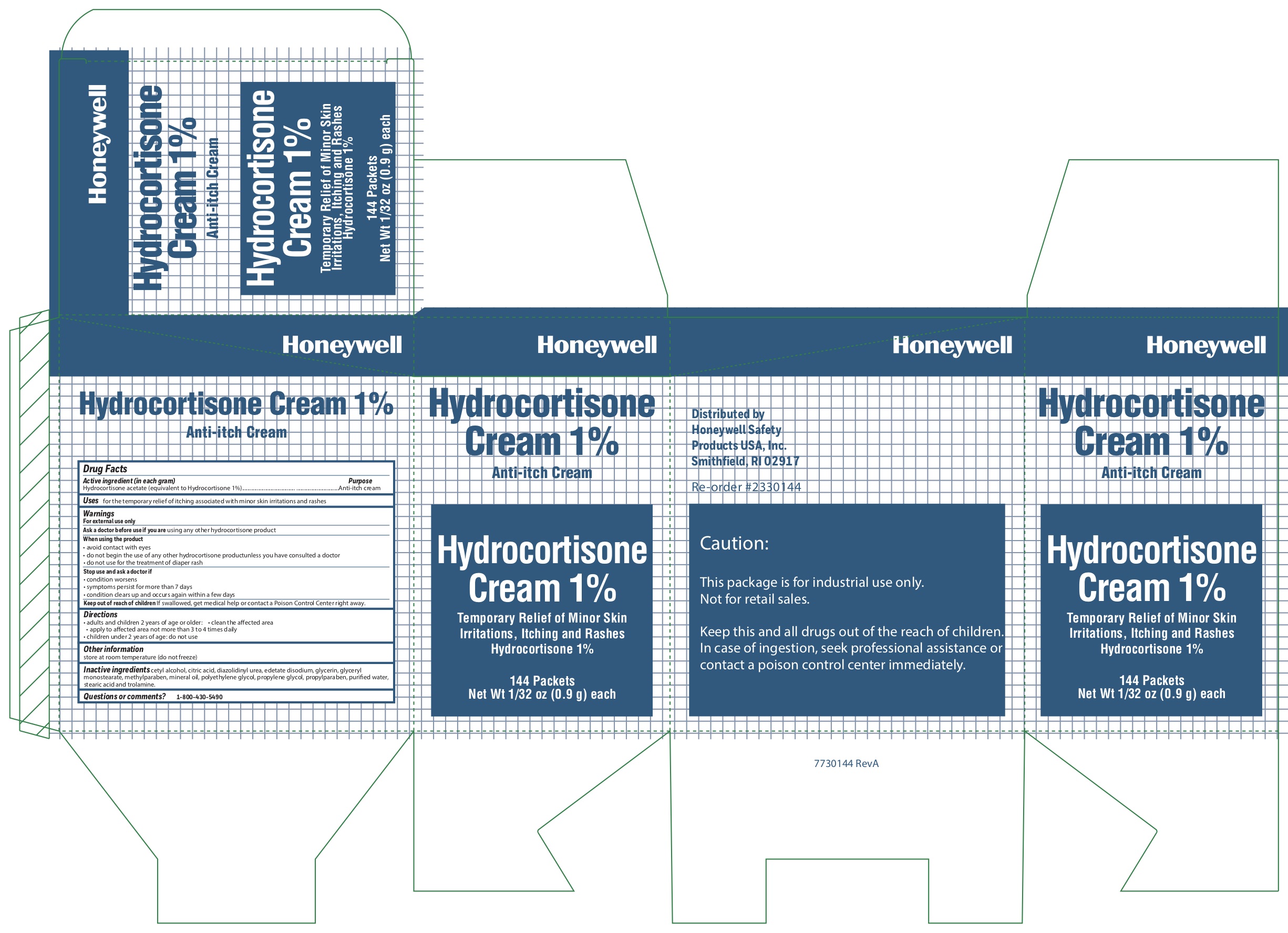
Hydrocortisone
Active ingredient (in each gram)
Hydrocortisone acetate (equivalent to Hydrocortisone 1%)
Hydrocortisone
Uses
- for the temporary relief of itching associated with minor skin irritations and rashes
Hydrocortisone
Warnings
For external use only
When using the product
- avoid contact with eyes
- do not begin use of any other hydrocortisone product unless you have consulted a doctor
- do not use for the treatment of diaper rash
Hydrocortisone
Directions
- adults and children 2 years and older:
- clean the affected area
- apply to the area not more than 3 to 4 times daily
- children under 2 years of age: consult a doctor
Hydrocortisone
Inactive ingredients
cetyl alcohol, citric acid, diazolidinyl urea, edetate disodium, glycerin, glyceryl monostearate, methylparaben, mineral oil, polyethylene glycol, propylene glycol, propylparaben, purified water, stearic acid, trolamine
BZK
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
BZK
Other information
- store at room temperature 15 0 to 30 0 C (59 0 - 86 0 F)
- do not reuse towelette
4129
SF00004226 Kit Contents
1 AMMONIA INHALANTS 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
1 TRIANGULAR BDG, NON-STERILE
1 INSTANT COLD PACK 4" X 6"
1 ALCOHOL PREP PADS 10P
1 HYDROCORTISON,1.O%,1/32 OZ,10P
1 RESPONSE KIT BLOODBORNE PATHOG
1 SCISSOR BDGE 4" RED PLS HDL
1 BANDAGE COMP 2" W/TELFA PAD 4
1 BANDAGE COMP 4" W/TELFA PAD 1
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 PR LRG NITRILE GLVES ZIP BAG
1 1" X 3" PLASTIC BANDS 16/BAG
1 KIT STL 24 UN WHITE 01
1 PVP IODINE SWABS 10
1 STING Relief SWAB 10
1 PYRO-CAINE AERO 2/BX
1 RED BIO BAGS 2/BX
1 FACE MASK/EYE SHIELD
1 LIQD TRTMNT SYS 1 EA
1 DISP. TOWEL/WIPES 2EA
1 IMPERVIOUS GOWN 1 EA
| 4129 FIRST AID KIT
4129 first aid kit kit |
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| Labeler - Honeywell Safety Products USA, INC (118768815) |