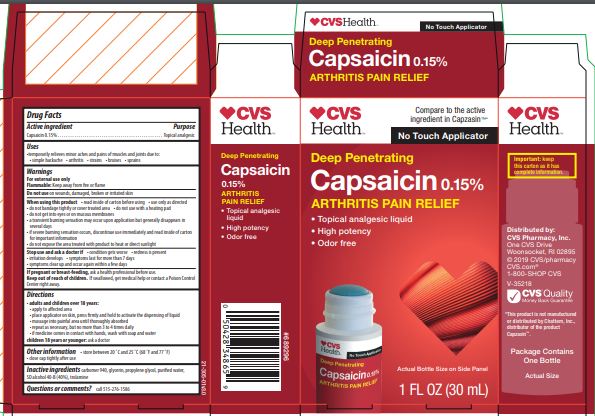
Active Ingredient
Capsaicin 0.15%........................................................................................................................... Topical analgesic
Uses
temporarily relieves minor aches and pains of muscles and joints due to:
simple backache
arthritis
strains
bruises
sprains
warnings
For external use only
When using this product
use only as directed
do not bandage tightly or cover treated area
do not use with a heating pad
do not get into eyes or on mucous membranes
a transient burning sensation may occur upon application but generally disappears in several days
if severe burning sensation occurs, discontinue use immediately and read inside of carton for important information
do not expose the area treated with product to heat or direct sunlight
Directions
apply to affected area
place applicator on skin, press firmly and hold to activate the dispensing of liquid
massage into painful area until thoroughly absorbed
repeat as necessary, but no more than 3 to 4 times daily
if medicine comes in contact with hands, wash with soap and water
PLEASE READ AND KEEP THESE DIRECTIOINS
What is capsaicin? Capsaicin, a naturally occuring substance derived from hot peppers, is a safe and effective topical analgesic for arthritis pain.
What does capsaicin do? When applied to the affected area, capsaicin penetrates deep and specically targets pain transmitting neurons by progressively deteriorating their ability to signal pain to the brain, effectively relieving minor aches and pains of muscles and joints associated with arthritis, simple backache, strains, sprains and bruises.
When used every day, as directed, the effectiveness of capsaicin continues to build more and more. The amount of time required for pain relief to occur varies with each person. Some arthritis sufferers may obtain relief within the rst week of use, while others may experience relief later. This product can be used for all types of arthritis.
Are there any side effects? Due to the nature of capsaicin, a mild tolerable burning and/or itching sensation may be experienced when the product is applied which may last up to 48 hours. This mild burning and/or itching sensation typically diminishes with continued use, and is not a reason to discontinue using this product.
Severe discomfort has been reported in some individuals. If severe burning and/or itching occurs, discontinue use immediately and remove excess product by thoroughly washing with soap and cold water. If regular soap and water does not completely wash away the residue, try using dishwashing liquid or cooking oil at room temperature. This discomfort can be expected to subside completely. If you experience blistering, or signicant improvement does not occur, stop use and contact your doctor.
Warm or hot water, direct sunlight or exposure to heat may increase the likelihood of burning and itching. Therefore, do not apply immediately before or after activities such as bathing, swimming, using a hot tub, sunbathing or exposure to heat.
• If you are a rst time user and think your skin might be sensitive to capsaicin, test it on a small area rst.
• Children 18 years of age or younger: consult a doctor before using.
• Wear gloves to apply or, if medicine comes in contact with hands, wash with soap and water after applying to avoid spreading to the eyes or other sensitive areas of the body. Try using dishwashing liquid or cooking oil at room temperature if regular soap and water does not completely wash the product from your hands. If product gets into the eyes, ush with water.
• Apply to affected area and using the applicator, press rmly on skin and hold to activate the dispensing of liquid. Massage into painful area until thoroughly absorbed.
• If you are using capsaicin on your hands, allow 30 minutes for it to penetrate before washing. During this time avoid touching damaged or irritated skin and avoid contact with the eyes and mucous membranes. After 30 minutes, wash hands with soap and water.
• If you wear contact lenses, avoid touching them after applying this product. Handle lenses before use.