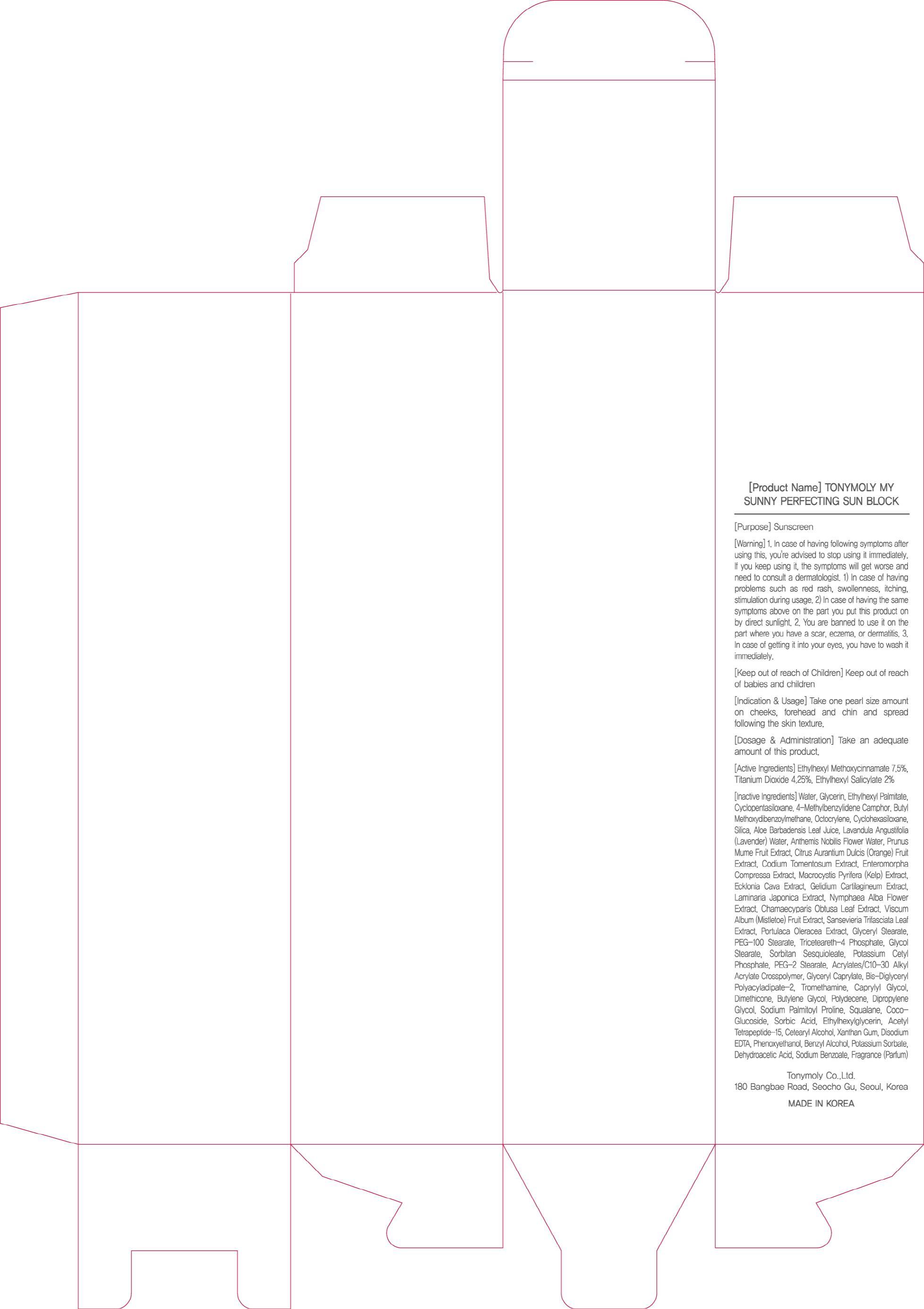
ACTIVE INGREDIENT
Active Ingredients: Ethylhexyl Methoxycinnamate 7.5%, Titanium Dioxide 4.25%, Ethylhexyl Salicylate 2%
INACTIVE INGREDIENT
Inactive Ingredients:
Water, Glycerin, Ethylhexyl Palmitate, Cyclopentasiloxane, 4-Methylbenzylidene Camphor, Butyl Methoxydibenzoylmethane, Octocrylene, Cyclohexasiloxane, Silica, Aloe Barbadensis Leaf Juice, Lavandula Angustifolia (Lavender) Water, Anthemis Nobilis Flower Water, Prunus Mume Fruit Extract, Citrus Aurantium Dulcis (Orange) Fruit Extract, Codium Tomentosum Extract, Enteromorpha Compressa Extract, Macrocystis Pyrifera (Kelp) Extract, Ecklonia Cava Extract, Gelidium Cartilagineum Extract, Laminaria Japonica Extract, Nymphaea Alba Flower Extract, Chamaecyparis Obtusa Leaf Extract, Viscum Album (Mistletoe) Fruit Extract, Sansevieria Trifasciata Leaf Extract, Portulaca Oleracea Extract, Glyceryl Stearate, PEG-100 Stearate, Triceteareth-4 Phosphate, Glycol Stearate, Sorbitan Sesquioleate, Potassium Cetyl Phosphate, PEG-2 Stearate, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Glyceryl Caprylate, Bis-Diglyceryl Polyacyladipate-2, Tromethamine, Caprylyl Glycol, Dimethicone, Butylene Glycol, Polydecene, Dipropylene Glycol, Sodium Palmitoyl Proline, Squalane, Coco-Glucoside, Sorbic Acid, Ethylhexylglycerin, Acetyl Tetrapeptide-15, Cetearyl Alcohol, Xanthan Gum, Disodium EDTA, Phenoxyethanol, Benzyl Alcohol, Potassium Sorbate, Dehydroacetic Acid, Sodium Benzoate, Fragrance(Parfum)
WARNINGS
Warning:
1. In case of having following symptoms after using this, you're advised to stop using it immediately. If you keep using it, the symptoms will get worse and need to consult a dermatologist.
1) In case of having problems such as red rash, swollenness, itching, stimulation during usage.
2) In case of having the same symptoms above on the part you put this product on by direct sunlight.
2. You are banned to use it on the part where you have a scar, eczema, or dermatitis.
3. In case of getting it into your eyes, you have to wash it immediately.