VITAFOL FE PLUS- vitamin a, ascorbic acid, vitamin d, .alpha.-tocopherol, thiamine mononitrate, riboflavin, niacin, pyridoxine hydrochloride, folic acid, cyanocobalamin, iodine, iron, magnesium, zinc, copper, doconexent, and docusate sodium
Exeltis USA, Inc.
Disclaimer: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
----------
Vitafol
® Fe
+ Supplement
Prenatal Supplement with 90 mg Iron, and Optional Stool Softener
COMPOSITION
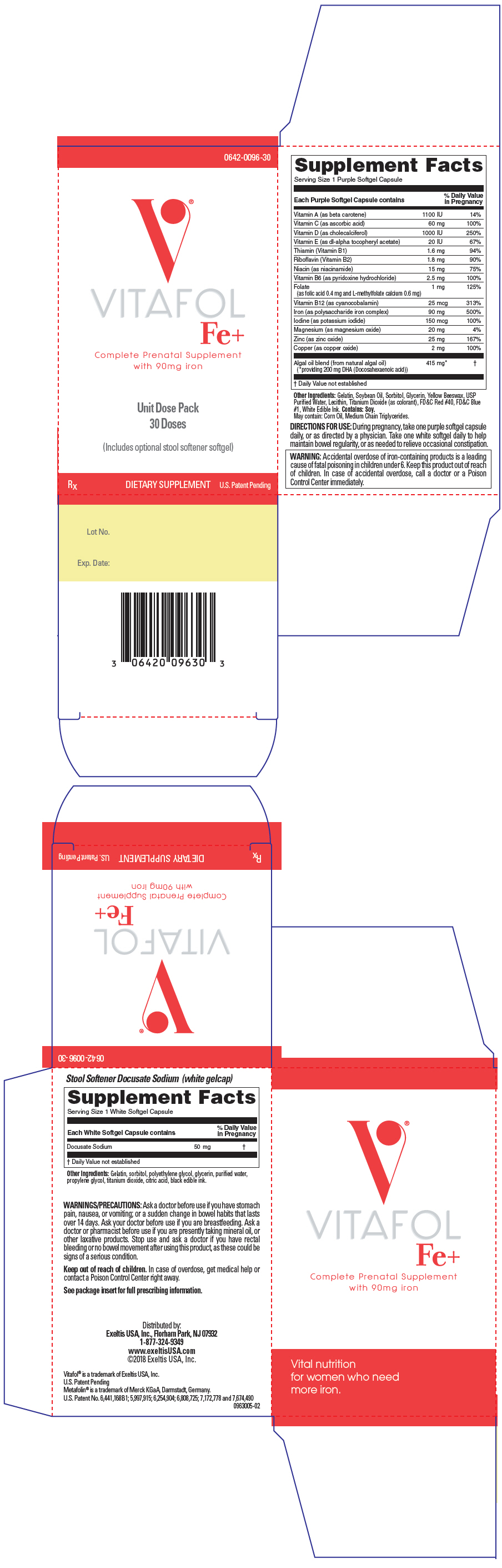
Each PURPLE softgel capsule contains:
| Vitamin A (as beta carotene) | 1100 IU |
| Vitamin C (as ascorbic acid) | 60 mg |
| Vitamin D (as cholecalciferol) | 1000 IU |
| Vitamin E (as dl-alpha tocopheryl acetate) | 20 IU |
| Thiamin (Vitamin B1) | 1.6 mg |
| Riboflavin (Vitamin B2) | 1.8 mg |
| Niacin (as niacinamide) | 15 mg |
| Vitamin B6 (as pyridoxine hydrochloride) | 2.5 mg |
| Folate | 1 mg |
| (as Folic acid USP 0.4 mg; as L-methylfolate calcium 0.6 mg) | |
| Vitamin B12 (as cyanocobalamin) | 25 mg |
| Iron (as polysaccharide iron complex) | 90 mg |
| Iodine (as potassium iodide) | 150 mcg |
| Magnesium (as magnesium oxide) | 20 mg |
| Zinc (as zinc oxide) | 25 mg |
| Copper (as copper oxide) | 2 mg |
| Algal oil blend (derived from Natural Algal Oil) | 415 mg |
| (*providing 200 mg DHA (docosahexaenoic acid)) | |
Other Ingredients: Gelatin, Soybean Oil, Sorbitol, Glycerin, Yellow Beeswax, USP Purified Water, Lecithin, Titanium Dioxide (as colorant), FD&C Red #40, FD&C Blue #1, White Edible Ink.
Contains: Soy. May also contain: Corn Oil, DL alpha-tocopherol, Medium ChainTriglycerides.
USAGE
Vitafol ® Fe + prenatal supplement provides vitamin, mineral and omega-3 fatty acid supplementation throughout pregnancy, including individuals with known allergies to fish. Vitafol ® Fe +does not contain fish, fish oils, fish proteins, or fish by-products.
CONTRAINDICATIONS
Vitafol ® Fe + prenatal supplement is contraindicated in patients with hypersensitivity to any of its components or color additives.
Folic acid is contraindicated in patients with untreated and uncomplicated pernicious anemia, and in those with anaphylactic sensitivity to folic acid.
Iron supplementation is contraindicated in patients with hemochromatosis and patients with iron storage disease or the potential for iron storage disease due to chronic hemolytic anemia (e.g., inherited anomalies of hemoglobin structure or synthesis and/or red cell enzyme deficiencies, etc.), pyridoxine responsive anemia, or cirrhosis of the liver.
Cyanocobalamin is contraindicated in patients with sensitivity to cobalt or to cyanocobalamin (vitamin B12).
WARNING
Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or a Poison Control Center immediately.
WARNINGS/PRECAUTIONS
This product is intended for use as directed by your healthcare provider. Do not share with others. Vitafol ® Fe + must be used with caution in patients with known sensitivity or allergy to soy.
Vitamin D supplementation should be used with caution in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones. High doses of vitamin D can lead to elevated levels of calcium that reside in the blood and soft tissues. Bone pain, high blood pressure, formation of kidney stones, renal failure, and increased risk of heart disease can occur.
Iodine should be used with caution in patients with an overactive thyroid.
Prolonged use of iron salts may produce iron storage disease.
Folic acid, especially in doses above 0.1 mg daily, may obscure pernicious anemia, in that hematologic remission may occur while neurological manifestations remain progressive. The use of folic acid doses above 1 mg daily may precipitate or exacerbate the neurological damage of vitamin B12 deficiency.
Consumption of more than 3 grams of omega-3 fatty acids per day from all sources may lead to excessive bleeding. Supplemental intake of omega-3 fatty acids such as DHA exceeding 2 grams per day is not recommended.
Avoid Overdosage. Keep out of the reach of children.
DRUG INTERACTIONS
Medications for an overactive thyroid (anti-thyroid drugs) used in conjunction with iodine supplementation may lead to hypothyroidism.
Medications for hypertension used in conjunction with iodine supplementation may increase potassium levels in blood.
High doses of folic acid may result in decreased serum levels of the anticonvulsant drugs; carbamazepine, fosphenytoin, phenytoin, phenobarbital, valproic acid. Folic acid may decrease a patient's response to methotrexate.
Vitamin D supplementation should not be given with large amounts of calcium in those with hypercalcemia or conditions that may lead to hypercalcemia such as hyperparathyroidism and those who form calcium-containing kidney stones.
Zinc can inhibit the absorption of certain antibiotics; take at least 2 hours apart to minimize interactions.
ADVERSE REACTIONS
Adverse reactions have been reported with specific vitamins and minerals, but generally at doses substantially higher than those in Vitafol ® Fe +. However, allergic and idiosyncratic reactions are possible at any dose. Reported adverse events include skin ailments, gastrointestinal complaints, glucose abnormalities, and visual problems.
COMPOSITION
Each WHITE softgel capsule contains: Docusate sodium, 50 mg
Other Ingredients: Gelatin, Sorbitol, Polyethylene Glycol, Glycerin, Purified Water, Propylene Glycol, Titanium Dioxide, Citric Acid, Black Edible Ink.
USAGE
Helps maintain bowel regularity and to provide relief from occasional constipation which may occur during pregnancy or associated with use of supplements containing iron.
WARNINGS/PRECAUTIONS
Ask a doctor before use if you have stomach pain, nausea, or vomiting; or a sudden change in bowel habits that lasts over 14 days.
Ask your doctor before use if you are breastfeeding.
Ask a doctor or pharmacist before use if you are presently taking mineral oil, or other laxative products.
Stop use and ask a doctor if you have rectal bleeding or no bowel movement after using this product, as these could be signs of a serious condition.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
DIRECTIONS FOR USE
Take one purple softgel capsule daily during pregnancy, or as directed by a physician.
Take one white softgel capsule daily, or as needed to help relieve occasional constipation. Take with water. May be taken at the same time as the prenatal supplement or separately.
HOW SUPPLIED
Vitafol ® Fe + is available as a purple, oval shaped softgel capsule imprinted "EX0096" and one white, oval shaped softgel capsule imprinted "50". Available in box of Unit-Dose pack of 30 (6 child resistant blister cards of 5+5 softgel capsules), Item No. 0642-0096-30 and as professional samples Item No. 0642-0096-01.
You should call your doctor for medical advice about adverse or unexpected reactions. To report to the company an adverse event or obtain product information, call 1-877-324-9349.
Distributed by:
Exeltis USA, Inc.
Florham Park, NJ 07932
1-877-324-9349
www.exeltisusa.com
©2018 Exeltis USA, Inc.
| These statements have not been evaluated by the Food and Drug Administration. This product is not intended to diagnose, treat, cure, or prevent any disease. |
Vitafol
® is a trademark of Exeltis USA, Inc.
Metafolin
® is a trademark of Merck KGaA, Darmstadt, Germany. U.S. Patent No. 6,441,168; 5,997,915; 6,254,904; 6,808,725, 7,172,778 and 7,674,490
Rev. November 2018
0963001-01
| VITAFOL FE PLUS
vitamin a, ascorbic acid, vitamin d, .alpha.-tocopherol, thiamine mononitrate, riboflavin, niacin, pyridoxine hydrochloride, folic acid, cyanocobalamin, iodine, iron, magnesium, zinc, copper, doconexent, and docusate sodium kit |
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Exeltis USA, Inc. (071170534) |
| Registrant - Exeltis USA, Inc. (071170534) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Exeltis USA, Inc. | 071170534 | label(0642-0096) | |