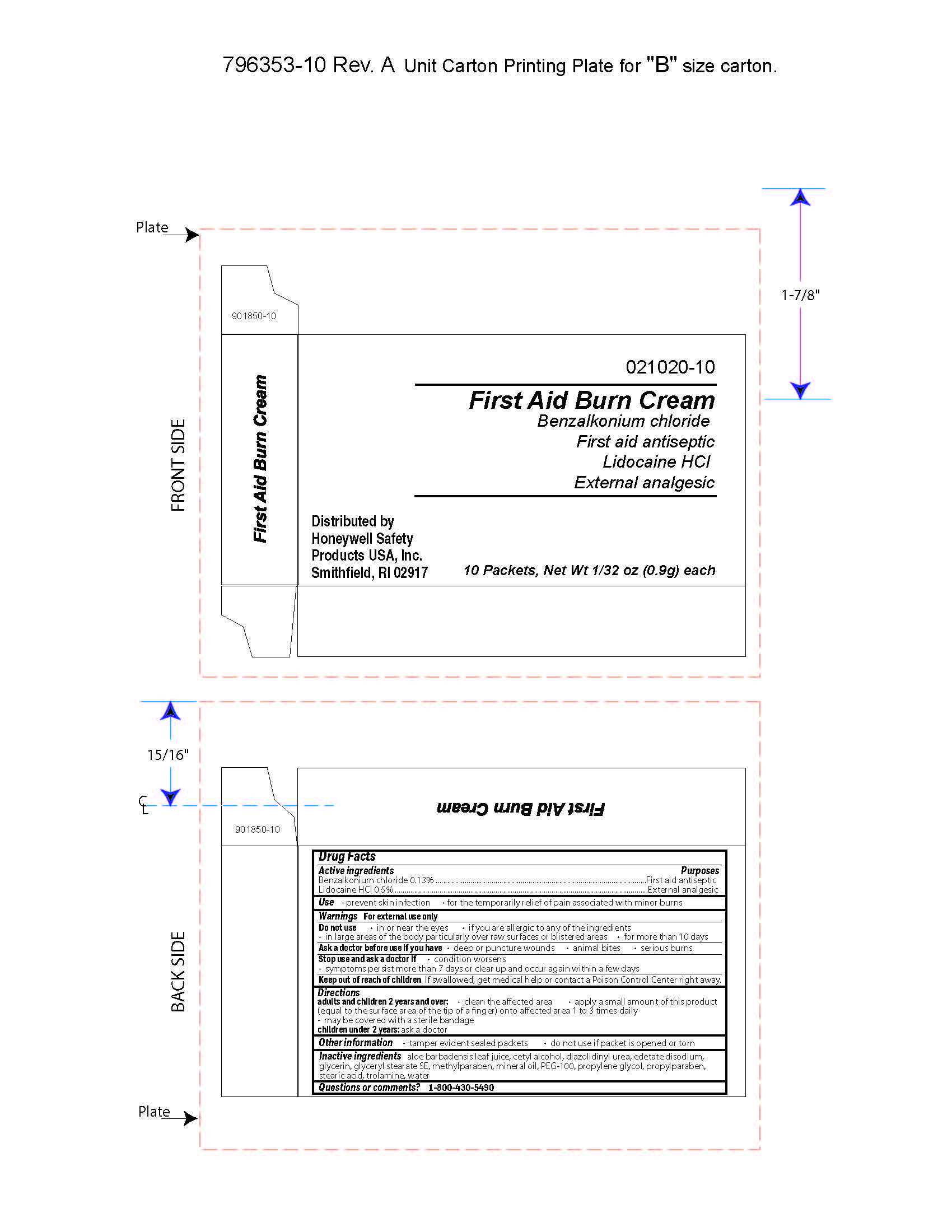
First Aid Burn Cream
Uses
- prevent skin infection
- for temporary relief of pain associated with minor burns
First Aid Burn Cream
Warnings
For external use only
First Aid Burn Cream
Directions
- adults and children 2 years of age and older:
- clean the affected area
- apply a small amount of this product (equal to the surface area of the tip of a finger) onto affected area 1 to 3 times daily
- may be covered with a sterile bandage
- children under 2 years of age: consult a doctor
First Aid Burn Cream
Other information
- tamper evident sealed packets
- do not use if packet is opened or torn
First Aid Burn Cream
Inactive ingredients
aloe barbadensis juice, cetyl alcohol, diazolidinyl urea, edetate disodium, glycerin, glyceryl stearate SE, methylparaben, mineral oil, PEG-100, propylene glycol, propylparaben, stearic acid, trolamine, water
Eyewash
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyewash
Warnings
For external use only Obtain immediate medical treatment for all open wounds in or near eyes. To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Eyewash
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
Aspirin
Active ingredient (in each tablet)
Aspirin 325 mg (NSAID)* *nonsteroidal anti-inflammatory drug
Aspirin
Uses
temporarily reduces fever and relieves minor aches and pains associated with:
- a cold
- headache
- toothache
- muscular aches
- backache
- minor pain of arthritis
- premenstrual and menstrual periods
Aspirin
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy alert: Aspirin may cause a severe allergic reaction which may include:
- hives
- facial swelling
- asthma (wheezing)
- shock
Stomach bleeding warning: This product contains an NSAID, which may cause severe stomach bleeding. The chance is higher if you:are:
- age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic drinks every day while using this product
- take more or for a longer time than directed
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis or kidney disease
- you are taking a diuretic
- you have asthma
Ask a doctor or pharmacist before use if you are
- taking a prescription drug for diabetes, gout or arthritis
Stop use and ask a doctor if
- you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- ringing in the ears or loss of hearing occurs
- any new symptoms appear
If pregnant or breast-feeding,
If pregnant or breat-feeding, ask a health professional before use. It is especially important not to use aspirin during the last three months of pregnancy unless definitely directed to do so by a doctor because it may cause problems in the unborn child or complications during delivery.
Aspirin
Directions
- drink a full glass of water with each dose
- adults and children 12 years of age and older: take 1 or 2 tablets every 4 hours while symptoms last, not more than 12 tablets in 24 hours
- children under 12 years of age: consult a doctor
Aspirin
Other information
- store at room temperature 15° - 30°C (59° - 86°F)
- TAMPER EVIDIENT PACKETS
- DO NOT USE IF OPEN OR TORN
Aspirin
Inactive ingredients
corn starch, croscarmellose sodium*, hypromellose*, microcrystalline cellulose*, mineral oil*, polyethylene glycol*, povidone, propylene glycol, silicon dioxide, stearic acid*, titanium dioxide*
*may contain these ingredients
BZK
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
BZK
Other information
- store at room temperature 15 0 to 30 0 C (59 0 - 86 0 F)
- do not reuse towelette
4134
SF00004169 Kit Contents
1 3/4 X 3 PLAS 100/BOX
1 TRIANGULAR BDG, NON-STERILE
1 INSTANT COLD PACK 4" X 6"
3 ANTIMCRBL ANTSPTC TWLETTS
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 TWEEZER PLASTICS 4"
1 GAUZE CLEAN-WRAP BDGE N/S 4"
1 ABD COMBINE PAD 5" X 9"
1 1 OZ, BUFF EYEWASH
1 SCISSOR BDGE 4" RED PLS HDL
1 # 25 EMPTY NO LOGO BLANK
1 F. A. INST CHART SM (INDIVIDUAL LBL)
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
2 PR LRG NITRILE GLVES ZIP BAG
6 FIRST AID CREAM 1.0GR PKT EACH
2 ADHES TAPE EYE STRIPS 2'S
2 EYE PADS STD OVAL STERILE
5 GAUZE PADS 4"X4" 12PLY
12 ASPIRIN BULK 2/PK