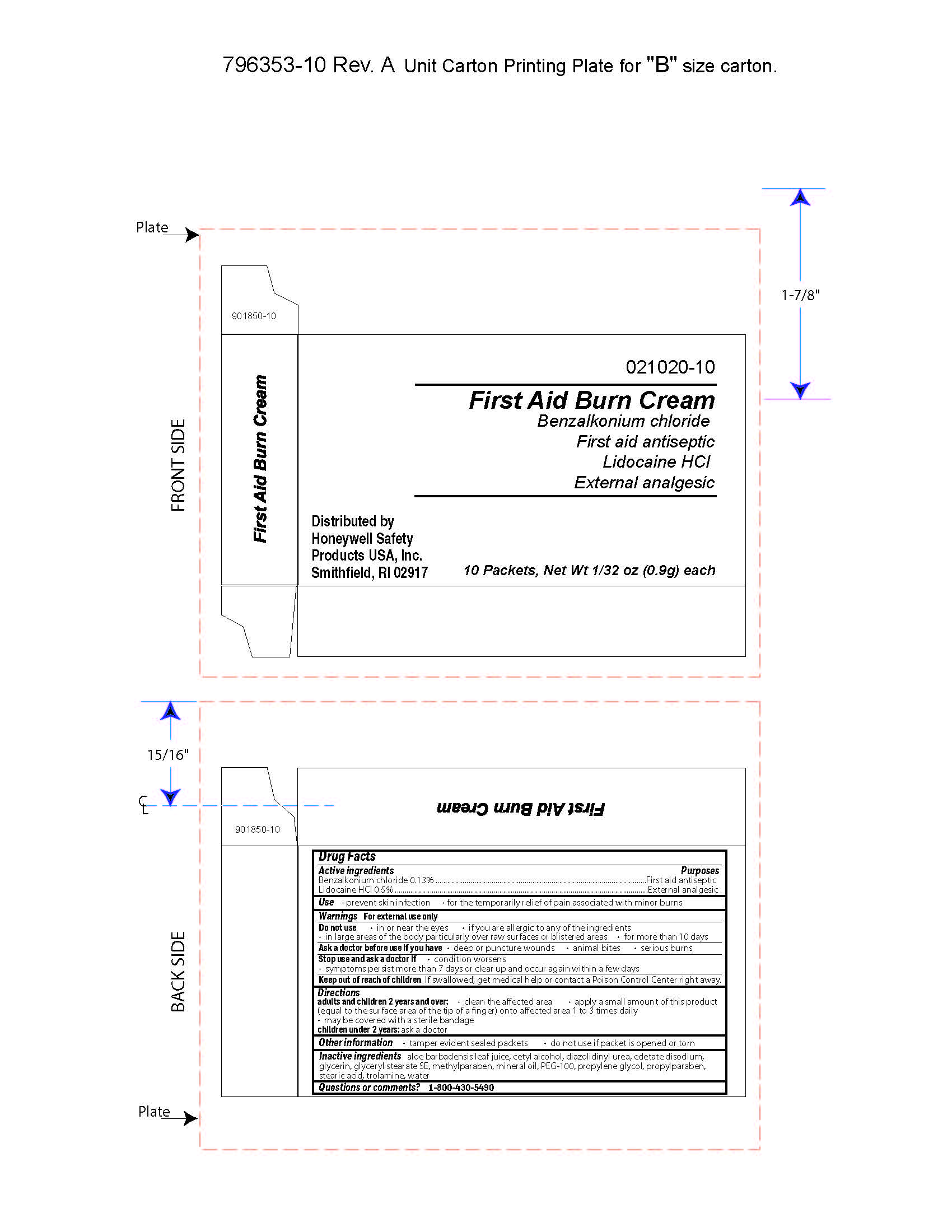
First Aid Burn Cream
Uses
- prevent skin infection
- for temporary relief of pain associated with minor burns
First Aid Burn Cream
Warnings
For external use only
First Aid Burn Cream
Directions
- adults and children 2 years of age and older:
- clean the affected area
- apply a small amount of this product (equal to the surface area of the tip of a finger) onto affected area 1 to 3 times daily
- may be covered with a sterile bandage
- children under 2 years of age: consult a doctor
First Aid Burn Cream
Other information
- tamper evident sealed packets
- do not use if packet is opened or torn
First Aid Burn Cream
Inactive ingredients
aloe barbadensis juice, cetyl alcohol, diazolidinyl urea, edetate disodium, glycerin, glyceryl stearate SE, methylparaben, mineral oil, PEG-100, propylene glycol, propylparaben, stearic acid, trolamine, water
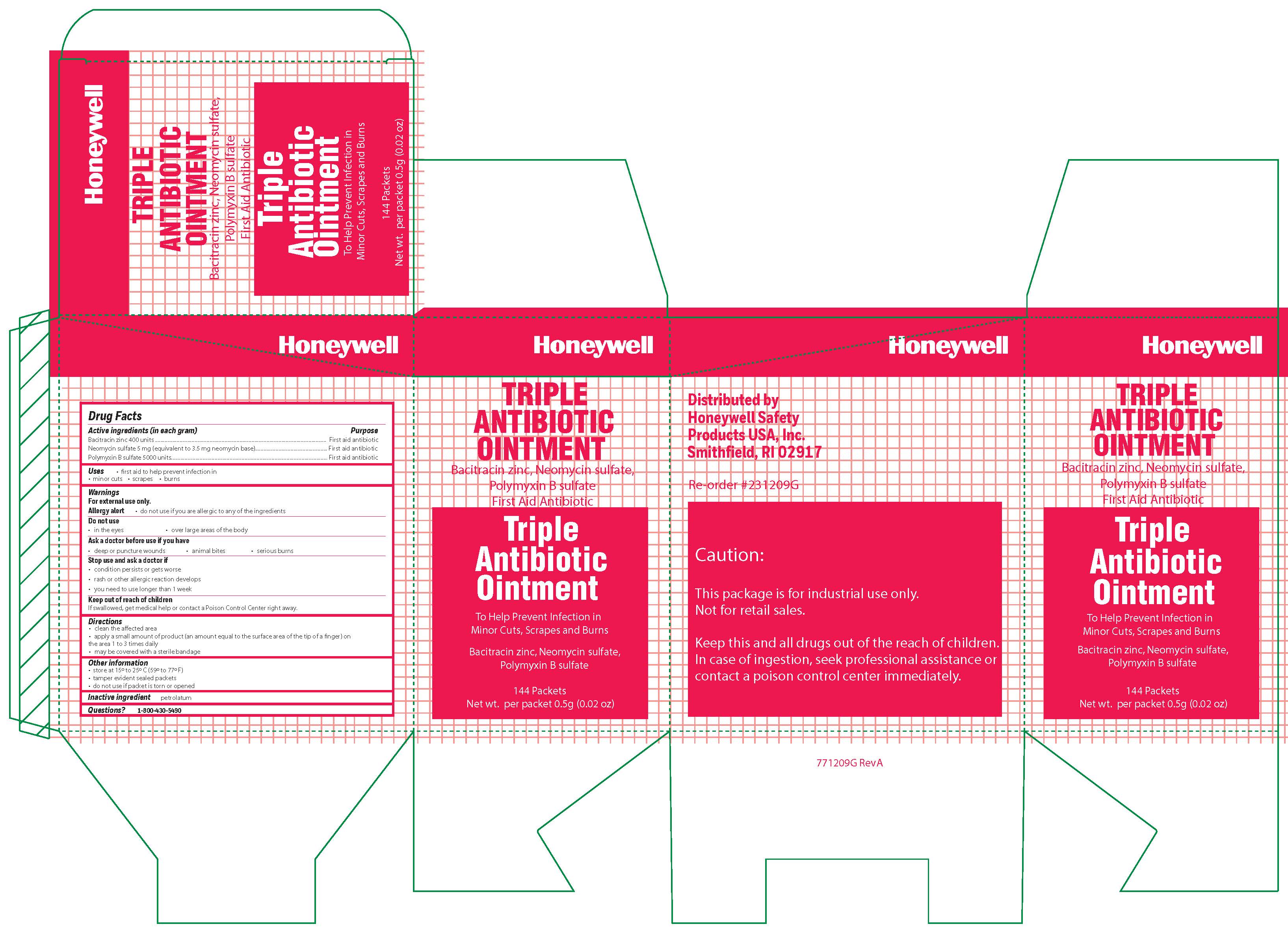
Triple
Active ingredient (each gram contains)
Bacitracin zinc 400 units - Neomycin sulfate (5 mg equivalent to 3.5 mg Neomycin base) Polymyxin B sulfate 5000 units
Triple
Warnings
For external use only
Allergy alert do not use if you are allergic to any of the ingredients
Triple
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Triple
Other information
store at 15 0 to 25 0 C (59 0 to 77 0 F) tamper evident sealed packets - do not use if packet is torn or opened
BZK
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
BZK
Other information
- store at room temperature 15 0 to 30 0 C (59 0 - 86 0 F)
- do not reuse towelette
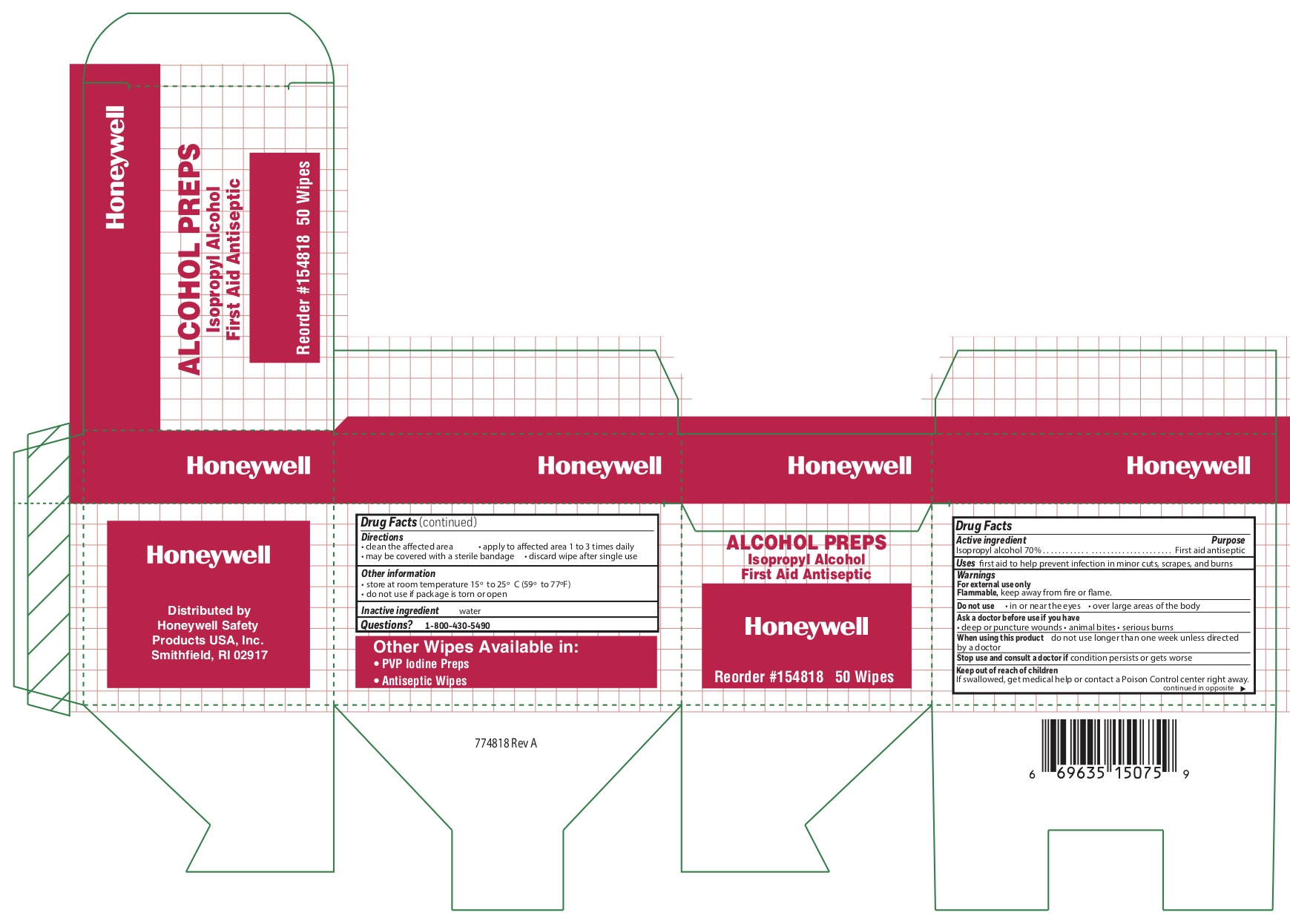
Alcohol Wipe
Directions
- clean the affected area
- may be covered with a sterile bandage
- apply wipe to affeted are 1 to 3 times daily
- discard wipe after single use
Alcohol Wipe
Other information
- store at room temperature 15 0 to 25 0 C (59 0 to 77 0 F)
- do not use if packet is torn or opened
4150
SF00003260 Kit Contents
1 TRIPLE ANTIBIOTIC 10 PER
1 FIRST AID BURN CREAM 6 PER
1 TRIANGULAR BDG, NON-STERILE
1 GAUZE PADS, 3" X 3", 4 PER
1 ADH TAPE, .5" X 2.5 YD, 2 PER
1 GAUZE COMP, 1 SQ YARD, 1 PER
1 ADHESIVE BDG,PLSTIC,1"X3"16PER
1 NITRILE GLOVES 2PR BBP
1 ANTIMCRBL ANTSPTC TWLETTS
1 FIRST AID GUIDE ASHI
1 ELASTIC BANDAGE 3" X 4.5YD
1 MICROSHIELD W/VNL GLV/ALCL
1 NORTH RESPONSE REFILL/KIT
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 KIT STL 24 UN WHITE 01
1 COLD PACK UNIT 4"X6" BULK