PRODUCT INFORMATION
Approved by FDA under NADA # 141-063
For intramuscular and subcutaneous use in beef and non-lactating dairy cattle only.
Not for use in female dairy cattle 20 months of age or older or in calves to be processed for veal.
DESCRIPTION NUFLOR Injectable Solution is a solution of the synthetic antibiotic florfenicol. Each milliliter of sterile NUFLOR Injectable Solution contains 300 mg of florfenicol, 250 mg N-methyl-2-pyrrolidone (NMP), 150 mg propylene glycol, and polyeth-ylene glycol qs. The chemical name for florfenicol is 2,2-Dichloro-N-[1-(fluoromethyl)-2-hydroxy-2-[4-(methylsulfonyl)phenyl]ethyl] acetamide.
INDICATIONS NUFLOR Injectable Solution is indicated for treatment of bovine respiratory disease (BRD) associated with Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni, and for the treatment of bovine interdigital phlegmon (foot rot, acute interdigital necrobacillosis, infectious pododermatitis) associated with Fusobacterium necrophorum and Bacteroides melaninogenicus. Also, it is indicated for the control of respiratory disease in cattle at high risk of developing BRD associated with Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni.
DOSAGE AND ADMINISTRATION For treatment of bovine respiratory disease (BRD) and bovine interdigital phlegmon (foot rot): NUFLOR Injectable Solution should be administered by intramuscular injection to cattle at a dose rate of 20 mg/kg body weight (3 mL/100 lbs). A second dose should be adminis-tered 48 hours later. Alternatively, NUFLOR Injectable Solution can be administered by a single subcutaneous (SC) injection to cattle at a dose rate of 40 mg/kg body weight (6 mL/100 lbs). Do not administer more than 10 mL at each site. The injection should be given only in the neck.
NOTE: Intramuscular injection may result in local tissue reaction which persists beyond 28 days. This may result in trim loss of edible tissue at slaughter. Tissue reaction at injection sites other than the neck is likely to be more severe.
For control of respiratory disease in cattle at high-risk of developing BRD: NUFLOR Injectable Solution should be administered by a single subcutaneous injection to cattle at a dose rate of 40 mg/kg body weight (6 mL/100 lbs). Do not administer more than 10 mL at each site. The injection should be given only in the neck.
| NUFLOR Injectable Solution DOSAGE GUIDE | ||||
| ANIMAL WEIGHT (lbs) | IM NUFLOR DOSAGE 3.0 mL/100 lb Body Weight (mL) | SC NUFLOR DOSAGE 6.0 mL/100 lb Body Weight (mL) | Recommended Injection Location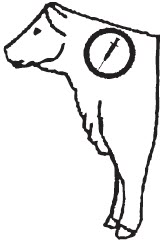 |
|
| 100 | 3.0 | 6.0 | ||
| 200 | 6.0 | 12.0 | ||
| 300 | 9.0 | 18.0 | ||
| 400 | 12.0 | 24.0 | ||
| 500 | 15.0 | 30.0 | ||
| 600 | 18.0 | 36.0 | Do not inject more than 10 mL per injection site. | |
| 700 | 21.0 | 42.0 | ||
| 800 | 24.0 | 48.0 | ||
| 900 | 27.0 | 54.0 | ||
| 1000 | 30.0 | 60.0 | ||
Clinical improvement should be evident in most treated subjects within 24 hours of initiation of treatment. If a positive response is not noted within 72 hours of initiation of treatment, the diagnosis should be re-evaluated.
USER SAFETY WARNINGS: NOT FOR HUMAN USE. KEEP OUT OF REACH OF CHILDREN. This product contains materials that can be irritating to skin and eyes. Avoid direct contact with skin, eyes, and clothing. In case of accidental eye exposure, flush with water for 15 minutes. In case of accidental skin exposure, wash with soap and water. Remove contaminated clothing. Consult a physician if irritation persists. Accidental injection of this product may cause local irritation. Consult a physician immediately. Reproductive and developmental toxicities have been reported in laboratory animals following high, repeated exposures to N-methyl-2-pyrrolidone (NMP). Pregnant women should wear gloves and exercise caution or avoid handling this product. The Safety Data Sheet (SDS) contains more detailed occupational safety information.
CONTACT INFORMATION: For customer service, adverse effects reporting and/or a copy of the SDS, call 1-800-211-3573. For additional information about adverse drug experience reporting for animal drugs, contact FDA at 1-888-FDA-VETS or www.fda.gov/reportanimalae.
PRECAUTIONS: Not for use in animals intended for breeding purposes. The effects of florfenicol on bovine reproductive performance, pregnancy, and lactation have not been determined. Toxicity studies in dogs, rats, and mice have associated the use of florfenicol with testicular degeneration and atrophy. Intramuscular injection may result in local tissue reaction which persists beyond 28 days. This may result in trim loss of edible tissue at slaughter. Tissue reaction at injection sites other than the neck is likely to be more severe.
RESIDUE WARNINGS: Animals intended for human consumption must not be slaughtered within 28 days of the last intramuscular treatment. Animals intended for human consumption must not be slaughtered within 38 days of subcutaneous treatment. This product is not approved for use in female dairy cattle 20 months of age or older, including dry dairy cows. Use in these cattle may cause drug residues in milk and/or in calves born to these cows. A withdrawal period has not been established in pre-ruminating calves. Do not use in calves to be processed for veal.
ADVERSE REACTIONS Inappetence, decreased water consumption, or diarrhea may occur transiently following treatment.
CLINICAL PHARMACOLOGY The pharmacokinetic disposition of NUFLOR Injectable Solution was evaluated in feeder calves following single intramuscular (IM) administration at the recommended dose of 20 mg/kg body weight. NUFLOR Injectable Solution was also administered intravenously (IV) to the same cattle in order to calculate the volume of distribution, clearance, and percent bioavailability1 (Table 1).
| Parameter | Median | Range |
|---|---|---|
| Cmax Maximum serum concentration Tmax Time at which Cmax is observed T ½ Biological half-life AUC Area under the curve Vdss Volume of distribution at steady state Clt Total body clearance |
||
| Cmax (μg/mL) | 3.07* | 1.43 - 5.60 |
| tmax (hr) | 3.33 | 0.75 - 8.00 |
| T ½ (hr) | 18.3† | 8.30 - 44.0 |
| AUC (μg∙min/mL) | 4242 | 3200 - 6250 |
| Bioavailability (%) | 78.5 | 59.3 - 106 |
| Vdss (L/kg)‡ | 0.77 | 0.68 - 0.85 |
| Clt (mL/min/kg)‡ | 3.75 | 3.17 - 4.31 |
Florfenicol was detectable in the serum of most animals through 60 hours after intramuscular administration with a mean concentration of 0.19 μg/mL. The protein binding of flor-fenicol was 12.7%, 13.2%, and 18.3% at serum concentrations of 0.5, 3.0, and 16.0 μg/mL, respectively.
MICROBIOLOGY Florfenicol is a synthetic, broad-spectrum antibiotic active against many Gram negative and Gram-positive bacteria isolated from domestic animals. It acts by binding to the 50S ribosomal subunit and inhibiting bacterial protein synthesis. Florfenicol is generally considered a bacteriostatic drug, but exhibits bactericidal activity against certain bacterial species. In vitro studies demonstrate that florfenicol is active against the bovine respiratory disease (BRD) pathogens Mannheimia haemolytica, Pasteurella multocida, and Histophilus somni, and that florfenicol exhibits bactericidal activity against strains of M. haemolytica and H. somni. Clinical studies confirm the efficacy of florfenicol against BRD as well as against commonly isolated bacterial pathogens in bovine inter-digital phlegmon including Fusobacterium necrophorum and Bacteroides melaninogenicus.
The minimum inhibitory concentrations (MICs) of florfenicol for BRD organisms were determined using isolates obtained from natural infections from 1990 to 1993. The MICs for interdigital phlegmon organisms were determined using isolates obtained from natural infections from 1973 to 1997 (Table 2).
| Indicated pathogens | Year of isolation | Isolate Numbers | MIC50†
(μg/mL) | MIC90†
(μg/mL) |
|---|---|---|---|---|
| Mannheimia haemolytica | 1990 to 1993 | 398 | 0.5 | 1 |
| Pasteurella multocida | 1990 to 1993 | 350 | 0.5 | 0.5 |
| Histophilis somni | 1990 to 1993 | 66 | 0.25 | 0.5 |
| Fusobacterium necrophorum | 1973 to 1997 | 33 | 0.25 | 0.25 |
| Bacteroides melaninogenicus | 1973 to 1997 | 20 | 0.25 | 0.25 |
ANIMAL SAFETY A 10× safety study was conducted in feeder calves. Two intramuscular injections of 200 mg/kg were administered at a 48-hour interval. The calves were monitored for 14 days after the second dose. Marked anorexia, decreased water consumption, decreased body weight, and increased serum enzymes were observed following dose administration. These effects resolved by the end of the study.
A 1×, 3×, and 5× (20, 60, and 100 mg/kg) safety study was conducted in feeder calves for 3× the duration of treatment (6 injections at 48-hour intervals). Slight decrease in feed and water consumption was observed in the 1× dose group. Decreased feed and water consumption, body weight, urine pH, and increased serum enzymes, were observed in the 3× and 5× dose groups. Depression, soft stool consistency, and dehydration were also observed in some animals (most fre-quently at the 3× and 5× dose levels), primarily near the end of dosing.
A 43-day controlled study was conducted in healthy cattle to evaluate effects of NUFLOR Injectable Solution administered at the recommended dose on feed consumption. Although a tran-sient decrease in feed consumption was observed, NUFLOR Injectable Solution administration had no long-term effect on body weight, rate of gain, or feed consumption.
STORAGE INFORMATION Store between 2°-30°C (36°-86°F). Refrigeration is not required. Protect from light when not in use. Use within 30 days of first puncture. For the 100mL vials, puncture the stopper a maximum of 3 times. For the 250mL and 500mL vials, puncture the stopper a maximum of 17 times.
HOW SUPPLIED NUFLOR Injectable Solution is packaged in 100 mL (NDC 0061-1116-04), 250 mL (NDC 0061-1116-05), and 500 mL (NDC 0061-1116-06) glass sterile multiple-dose vials.
REFERENCE 1. Lobell RD, Varma KJ, et al. Pharmacokinetics of florfenicol following intravenous and intramuscular doses to cattle. J Vet Pharmacol Therap. 1994;17:253-258.
Copyright© 2022 Merck & Co., Inc., Rahway, NJ, USA and its affiliates. All rights reserved.
Formulated in Germany.
Rev. 06/22
394942 R1
PRINCIPAL DISPLAY PANEL - 100 mL Vial Carton
100 mL
Multiple-Dose Vial
300 mg/mL
NDC 0061-1116-04
Sterile
Nuflor®
(FLORFENICOL)
Injectable Solution
For intramuscular and
subcutaneous use in beef
and non-lactating dairy cattle only.
Not for use in female dairy cattle
20 months of age or older or in
calves to be processed for veal.
Caution: Federal law restricts this
drug to use by or on the order of a
licensed veterinarian.
Approved by FDA under NADA # 141-063
MERCK
Animal Health