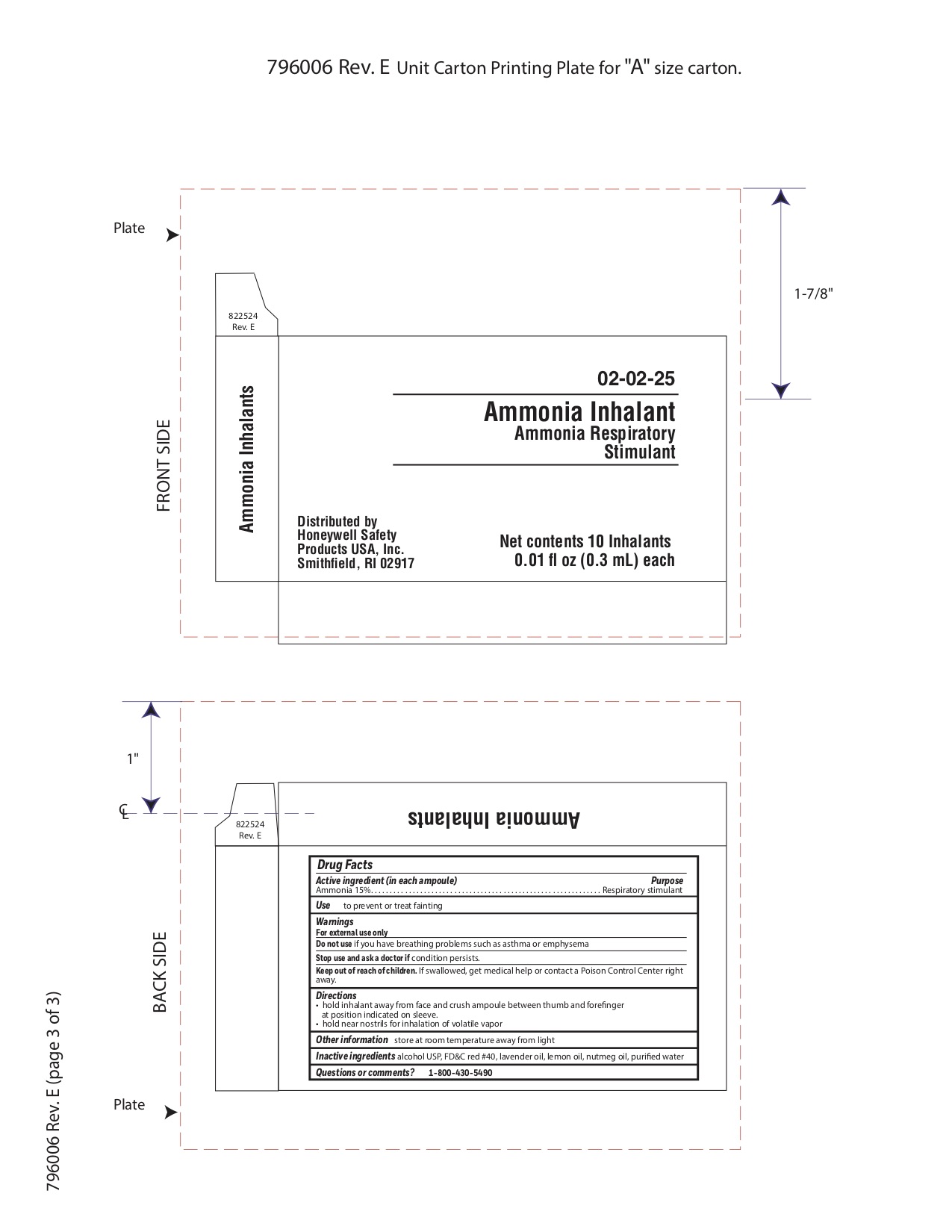
Keep out of reach of children
If swallowed get medical help or contact a Poison Control Center right away.
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Inactive ingredients
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
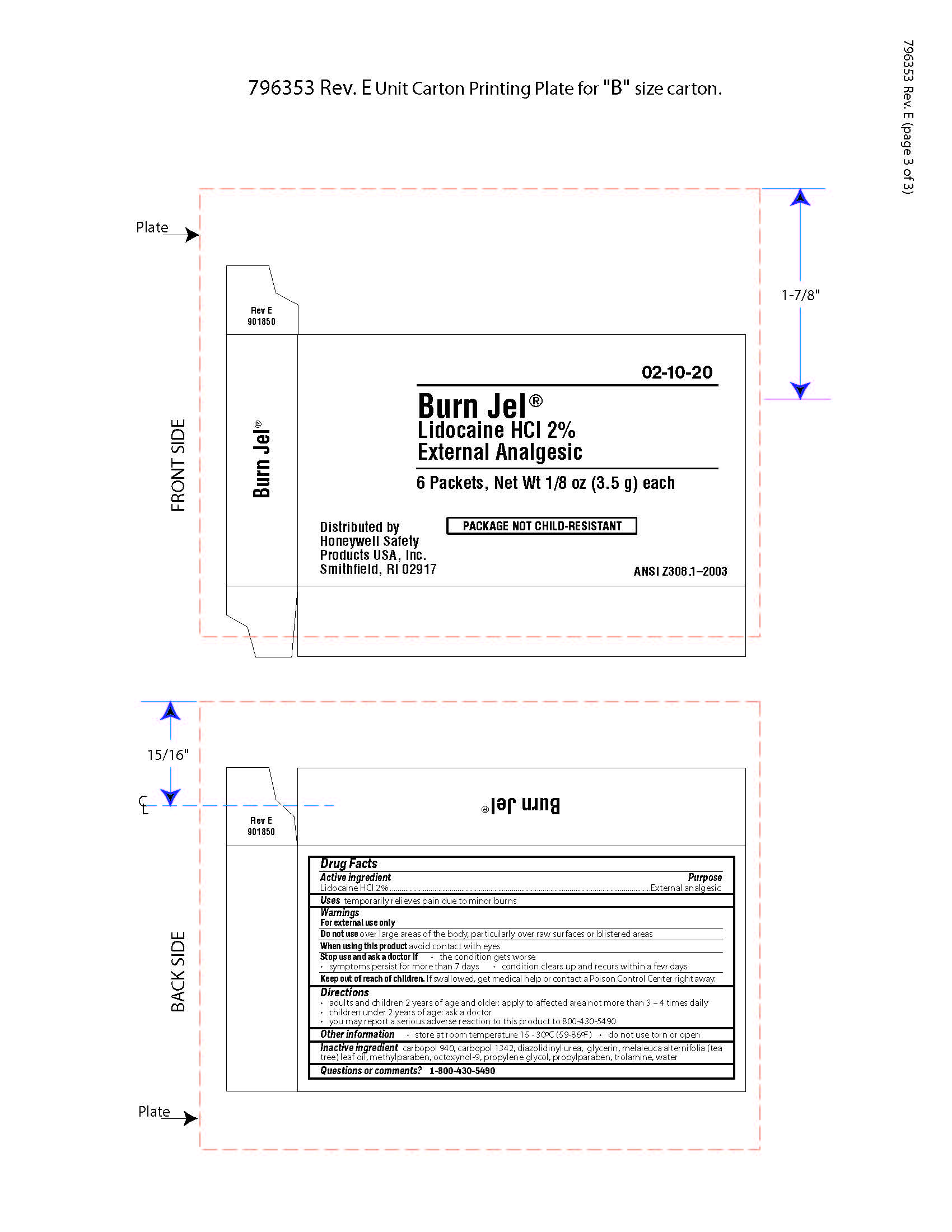
Burn Jel
Warnings
For external use only
Burn JEl
Directions
- adults and children 2 years of age and older; apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
- you may report a serious reaction to this product to 800-430-5490
Burn Jel
Inactive ingredients
carbopol 940, carbopol 1342, diazolidinyl urea, glycerin, melaleuca alternifolia (tea tree) leaf oil, methylparaben, octoxynol-9, propylene glycol, propylparaben, trolamine, water
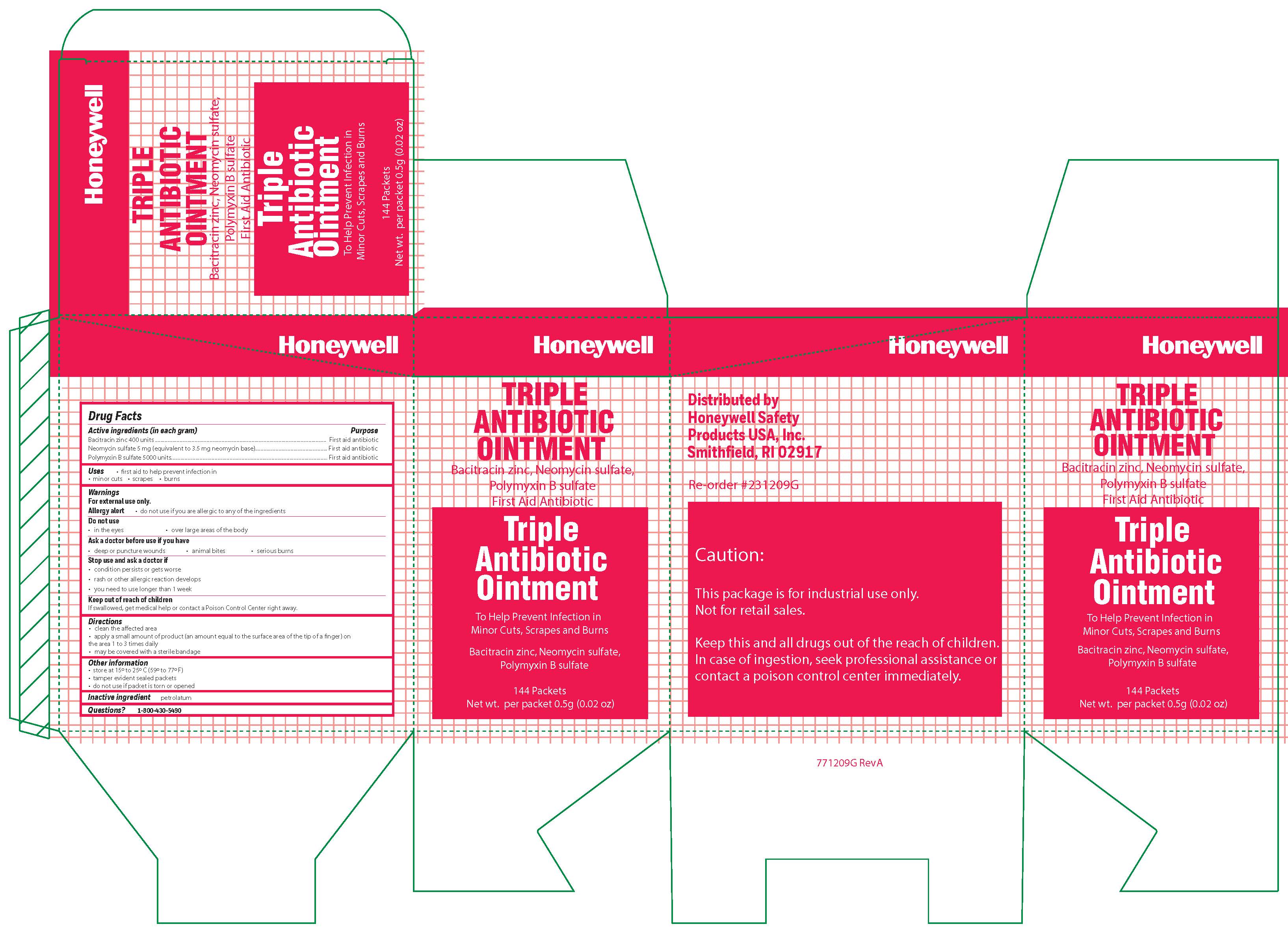
Triple
Active ingredient
Bacitracin zinc 400 units
Neomycin sulfate (5 mg equivalent to 3.5 mg Neomycin base)
Polymyxin B sulfate 5000 units
Triple
Warnings
For external use only
Allergy alert: do not use if you are allergic to any of the ingredients
Do not use
- in the eyes
- over large areas of the body
- Ask a doctor before use if you have
- a deep or puncture wounds
- animal bites
- serious burns
Triple
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Triple
Other information
- store at 15 0 to 25 0 C (59 0 to 77 0 F)
- tamper evident sealed packets
- do not use if packet is torn or opened
BZK Wipe
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
BZK Wipe
Other information
- store at room temperature 15 0 to 30 0 C (59 0 - 86 0 F)
- do not reuse towelette
Antihistamine
Uses
temporarily relieves these symptoms due to hay fever or other upper respiratory allergies
- runny nose
- sneezing
- itchy, watery eyes, itching of the nose or throat
Antihistamine
Warnings
Do not use
- with any other product containing diphenhydramine, even one used on the skin
Ask a doctor before use if you have
- glaucome
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor before use
- if child is taking a sedative or tranquilizer
- When using this product
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- use caution when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
Antihistamine
Directions
take every 4 to 6 hours
do not take more than 6 doses in 24 hours
adults and children 12 years of age and over 25 mg to 50 mg (1 to 2 tablets) not to exceed 300 mg in 24 hours
children 6 to under 12 years of age 12.5 mg** to 25 mg (1 tablet) not to exceed 150mg in 24 hours or as directed by a doctor
children under 6 years of age ask a doctor
**12.5 mg dosage strength is not available in this package
do not attempt to break tablets
Antihistamine
Inactive ingredients
carnuba wax, colloidal silicon dioxide, croscarmellose sodium D and C red no. 27 lake, dicalcium phosphate, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, stearic acid, titanium dioxide
eyewash
Uses
for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyewash
Warnings
For external use only-
Obtain immediate medical treatment for all open wounds in or near eyes.
To avoid contamination, do not touch tip of container to any surface.
Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Eyewash
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
4155
SF00000981 Kit Contents
1 TRIPLE ANTIBIOTIC 10 PER
1 AMMONIA INHALANTS 10 PER
2 TRIANGULAR BDG, NON-STERILE
1 GAUZE COMPRESS, 1728 SQ IN 1
1 GAUZE BANDAGE, 2" X 6 YD,2 PER
1 INSTANT COLD PACK 4" X 6"
4 ADHESIVE BDG,PLSTIC,1"X3"16PER
1 1 OZ EYE WASH W/PADS & STRIPS
1 BURN JEL 1/8 OZ, 6 PER
3 ANTIMCRBL ANTSPTC TWLETTS
1 FIRST AID GUIDE ASHI
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
1 BANDAGE COMP 2" W/TELFA PAD 4
2 BANDAGE COMP 4" W/TELFA PAD 1
LBL STOCK 6-3/8"X4"
1 LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 KIT STL 24 UN WHITE 01
1 WOVEN KNUCKLE 8'S
1 ADHS TAPE .5"X2.5YD 2
1 GAUZE PADS 3"X3" 4/BX
1 ANTIHISTAMINE BULK 1/PKK