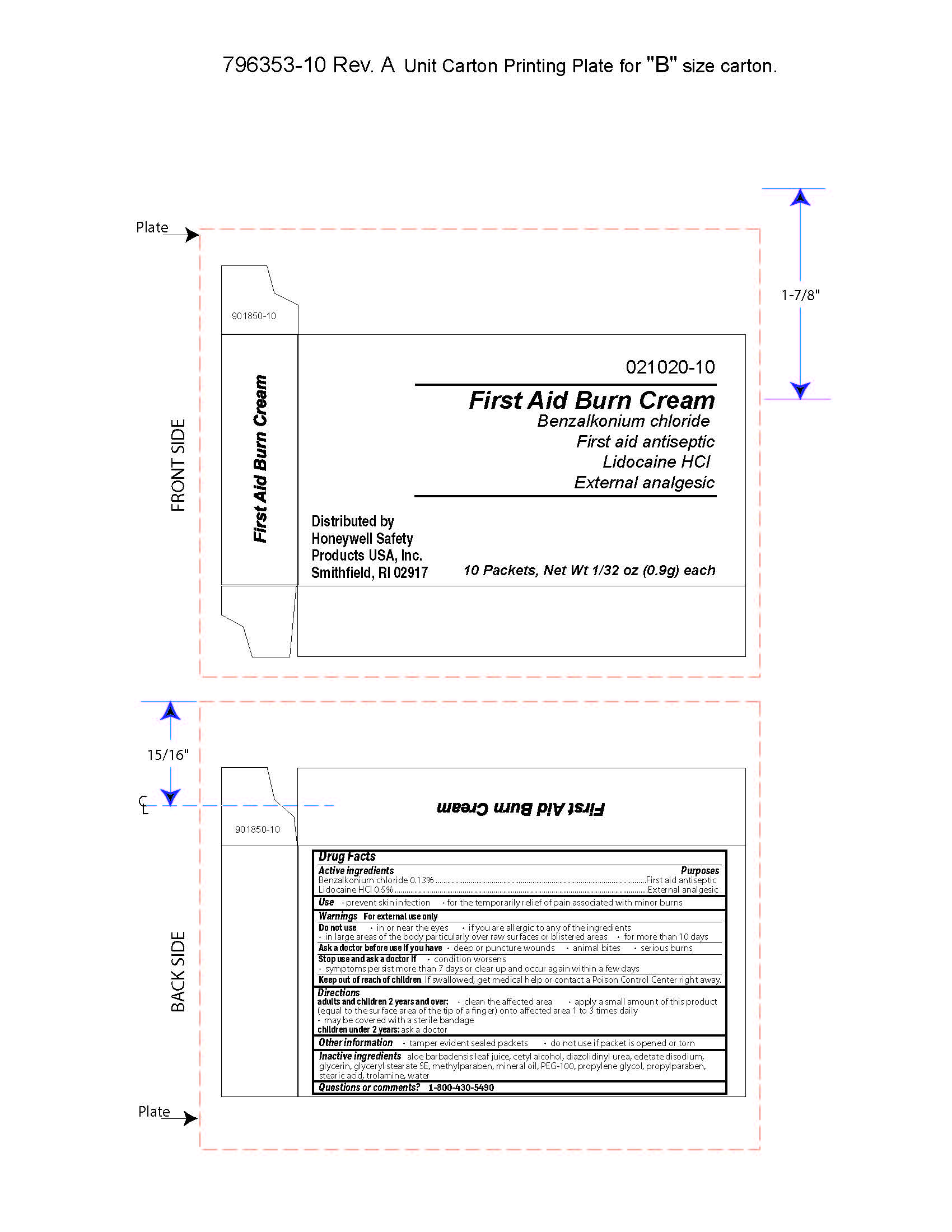
First Aid Burn Cream
Uses
- prevent skin infection
- for temporary relief of pain associated with minor burns
First Aid Burn Cream
Warnings
For external use only
First Aid Burn Cream
Directions
- adults and children 2 years of age and older:
- clean the affected area
- apply a small amount of this product (equal to the surface area of the tip of a finger) onto affected area 1 to 3 times daily
- may be covered with a sterile bandage
- children under 2 years of age: consult a doctor
First Aid Burn Cream
Other information
- tamper evident sealed packets
- do not use if packet is opened or torn
First Aid Burn Cream
Inactive ingredients
aloe barbadensis juice, cetyl alcohol, diazolidinyl urea, edetate disodium, glycerin, glyceryl stearate SE, methylparaben, mineral oil, PEG-100, propylene glycol, propylparaben, stearic acid, trolamine, water
BZK
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
BZK
Other information
- store at room temperature 15 0 to 30 0 C (59 0 - 86 0 F)
- do not reuse towelette
PVP
Warnings
For external use only.
PVP
Directions
- clean the affected area
- apply1 to 3 times daily
- may be covered with a sterile bandage
- if bandaged, let dry first
- discard wipe after single use
PVP
Other information
- do not use on individuals who are allergic or sensitive to iodine
- store at controlled temperature 59-86ºF (15-30ºC)
- do not use if pouch is open or torn
4156
SF00000670 Kit Contents
1 FIRST AID BURN CREAM 6 PER
1 TRIANGULAR BDG, NON-STERILE
1 GAUZE PADS, 3" X 3", 4 PER
1 ADH TAPE, .5" X 2.5 YD, 2 PER
1 GAUZE COMP, 1 SQ YARD, 1 PER
1 INSTANT COLD PACK 4" X 6"
1 EYEWASH BOTTLES 1 OZ, UNITIZED 2/BX
3 ADHESIVE BDG,PLSTIC,1"X3"16PER
1 PVP IODINE WIPES 10 PER
1 NITRILE GLOVES 2PR BBP
1 ANTIMCRBL ANTSPTC TWLETTS
1 F. A. INST CHART SM (INDIVIDUAL LBL)
1 BANDAGE COMP 4" W/TELFA PAD 1
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 KIT, PP 16 UNIT FA