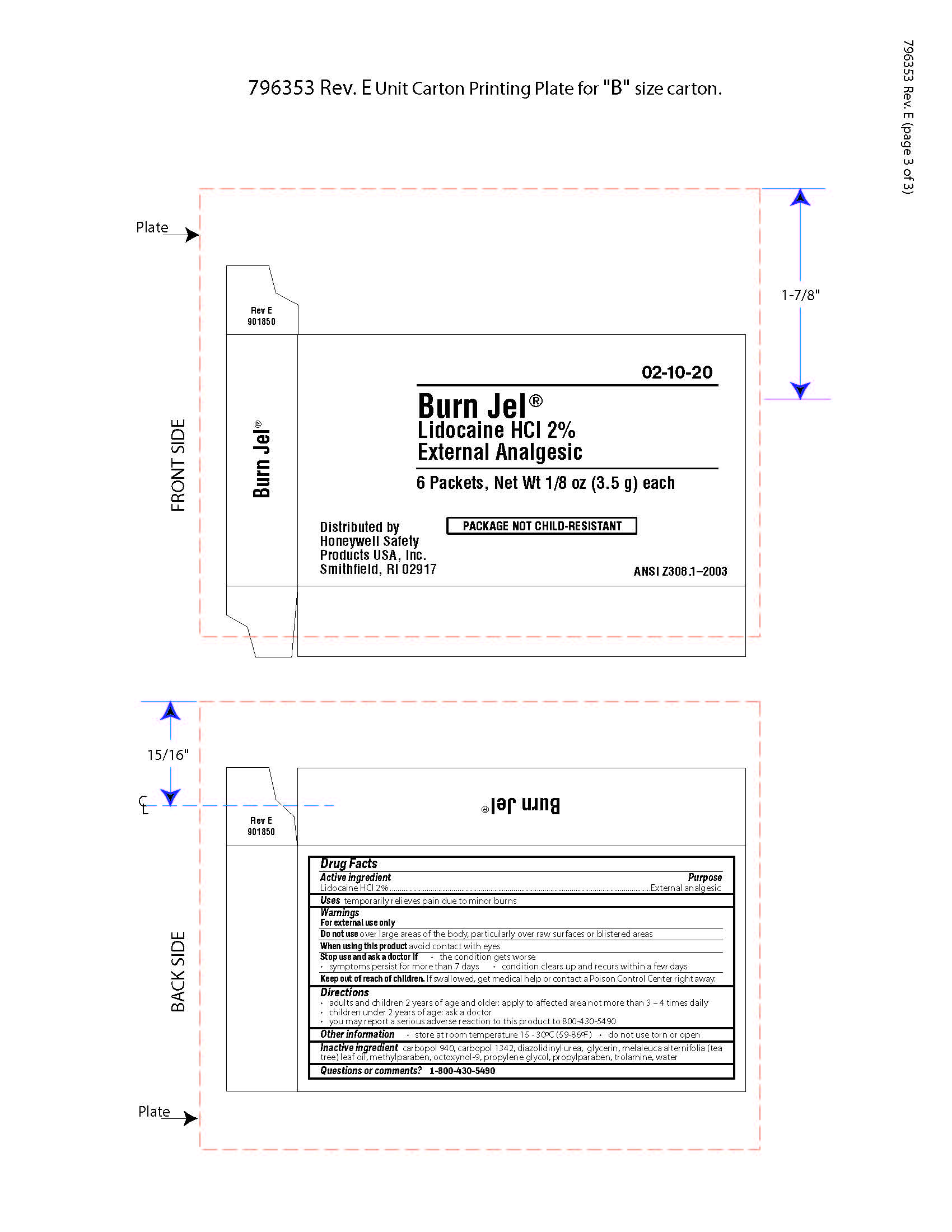
Burn Jel
Warnings
For external use only
Burn JEl
Directions
- adults and children 2 years of age and older; apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
- you may report a serious reaction to this product to 800-430-5490
Burn Jel
Inactive ingredients
carbopol 940, carbopol 1342, diazolidinyl urea, glycerin, melaleuca alternifolia (tea tree) leaf oil, methylparaben, octoxynol-9, propylene glycol, propylparaben, trolamine, water
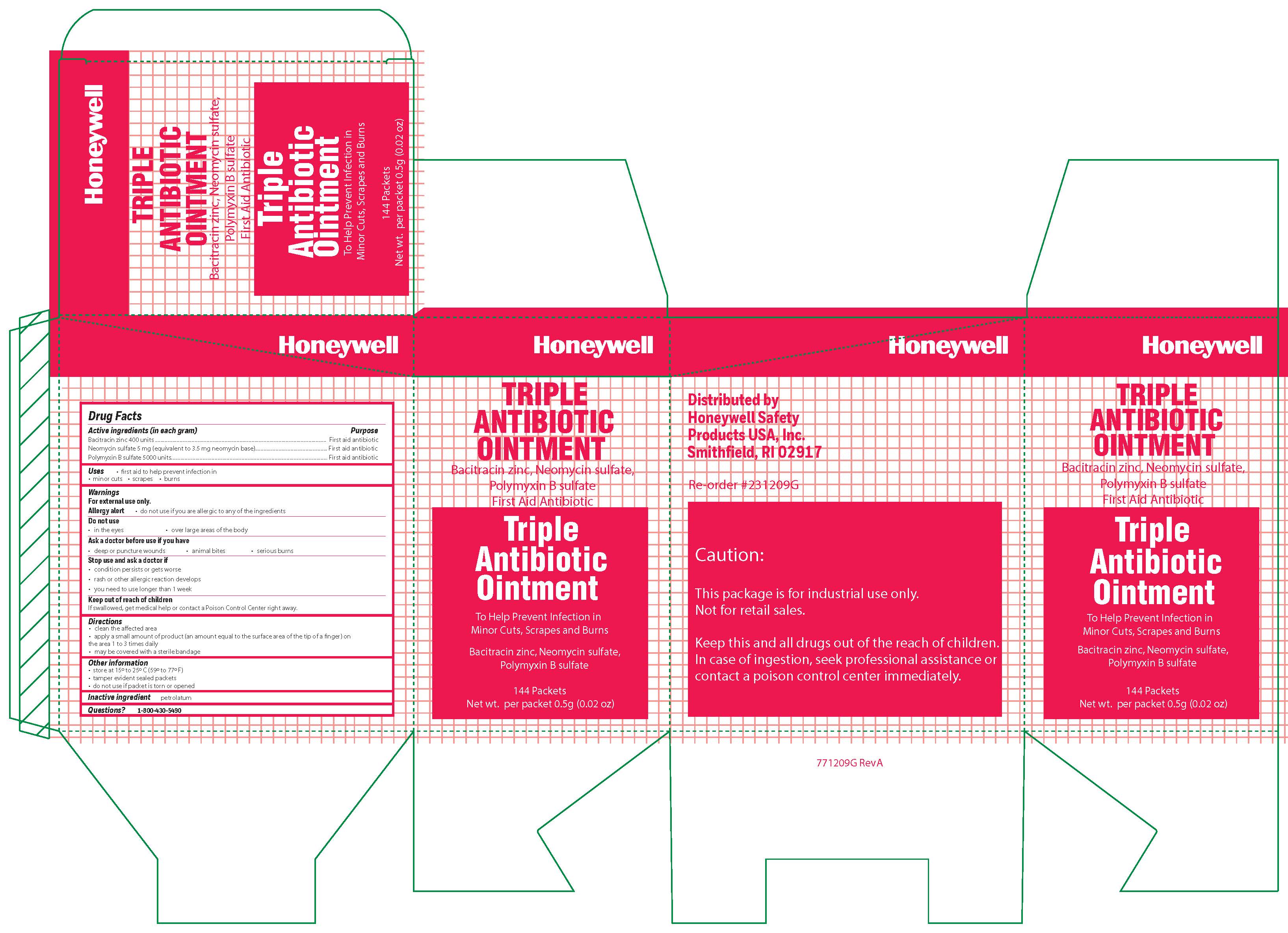
Triple
Active ingredient
Bacitracin zinc 400 units
Neomycin sulfate (5 mg equivalent to 3.5 mg Neomycin base)
Polymyxin B sulfate 5000 units
Triple
Warnings
For external use only
Allergy alert: do not use if you are allergic to any of the ingredients
Do not use
- in the eyes
- over large areas of the body
- Ask a doctor before use if you have
- a deep or puncture wounds
- animal bites
- serious burns
Triple
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Triple
Other information
- store at 15 0 to 25 0 C (59 0 to 77 0 F)
- tamper evident sealed packets
- do not use if packet is torn or opened
BZK Wipe
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
BZK Wipe
Other information
- store at room temperature 15 0 to 30 0 C (59 0 - 86 0 F)
- do not reuse towelette
Hand Sanitizer
Warnings
For external use only
Flammable, keep away from fire or flame
Hand Sanitizer
Inactive ingredients
acrylates/C10-30 alkyl acrylate crosspolymer, aloe barbadensis leaf juice, dl-alpha tocopheryl acetate, fragrance, PEG-60 almond glycerides, propylene glycol, purified water, triisopropanolamine
4252
68P2CCU Kit Contents
1 TRIPLE ANTIBIOTIC 10 PER
1 INSTANT COLD PACK 4" X 6"
1 BURN JEL 1/8 OZ, 6 PER
1 POR. CLOTH TAPE 2X10Yd
1 pOR. CLOTH TAPE 1/2X10Y
3 GAUZE CLEAN-WRAP BDGE N/S 4"
1 ABD COMBINE PAD 5" X 9"
1 ABD PADS 8"X10" STERILE
1 ELASTIC BANDAGE 3" X 4.5YD
1 CPR FILTERSHIELD 77-100
1 ANTISEPTIC WIPES BZK CHL 20'S
1 SCISSOR UTILITY SHEARS 7-1/4"
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
3 PR LRG NITRILE GLVES ZIP BAG
1 ANTISEPTIC HAND GEL 4OZ
1 WATER-JEL BURN DRESSING 4 X 4
1 KIT PP 24 UNIT FA
2 TRI BNDG NON WOVEN 40"X40"X56"
1 EYE PADS STD OVAL STERILE
1 GAUZE PADS 4"X4" 12PLY
5 WOVEN FINGERTIP BANDAGE 3"
10 HEAVY FLEX BANDAGE 7/8" X 3"
5 HEAVY FLEX KNUCKLE BANDAGE
5 HEAVY FLEX LARGE PATCH 2" X 3"
1 ZIP-LOCK BAG 5" X 5" .002