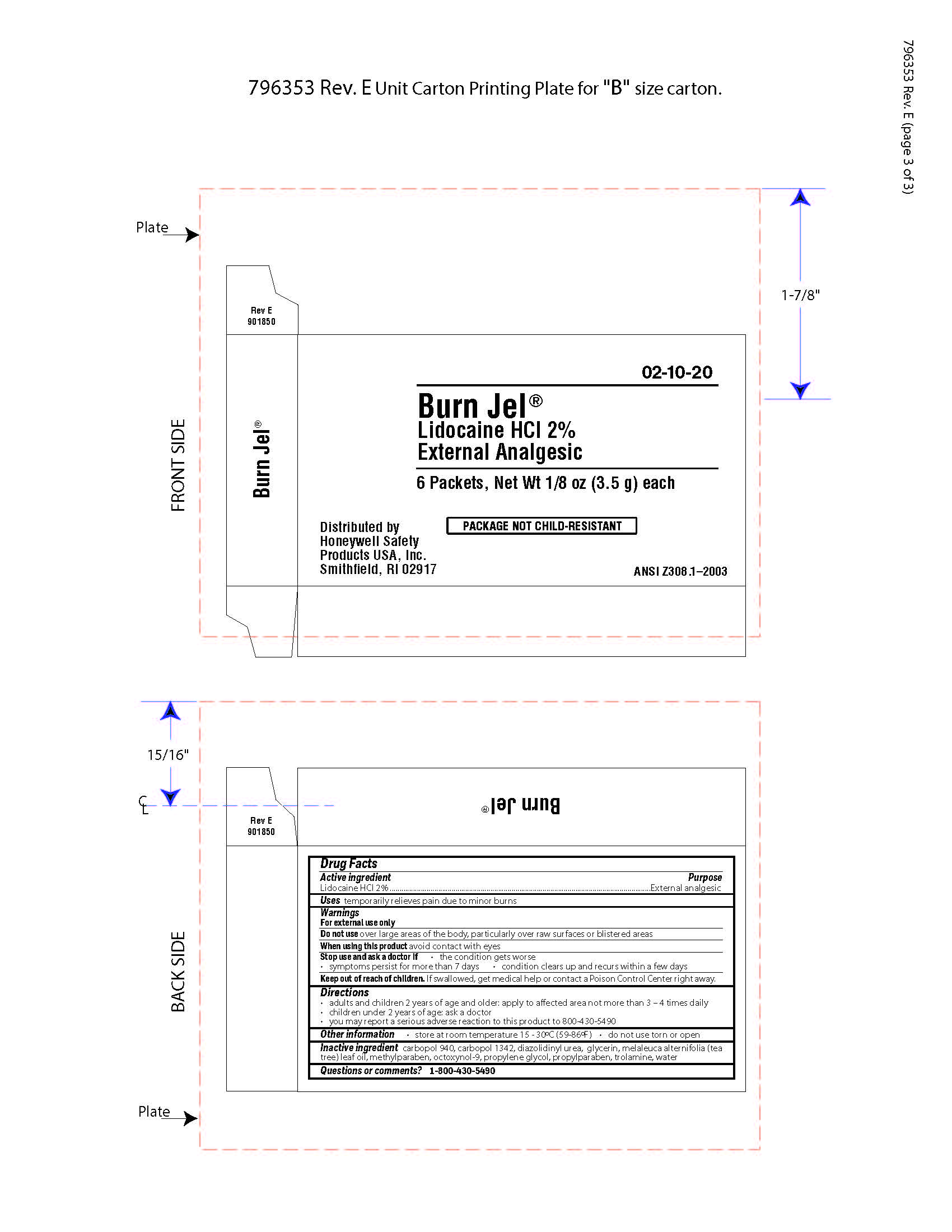
Burn Jel
Warnings
For external use only
Burn JEl
Directions
- adults and children 2 years of age and older; apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
- you may report a serious reaction to this product to 800-430-5490
Burn Jel
Inactive ingredients
carbopol 940, carbopol 1342, diazolidinyl urea, glycerin, melaleuca alternifolia (tea tree) leaf oil, methylparaben, octoxynol-9, propylene glycol, propylparaben, trolamine, water
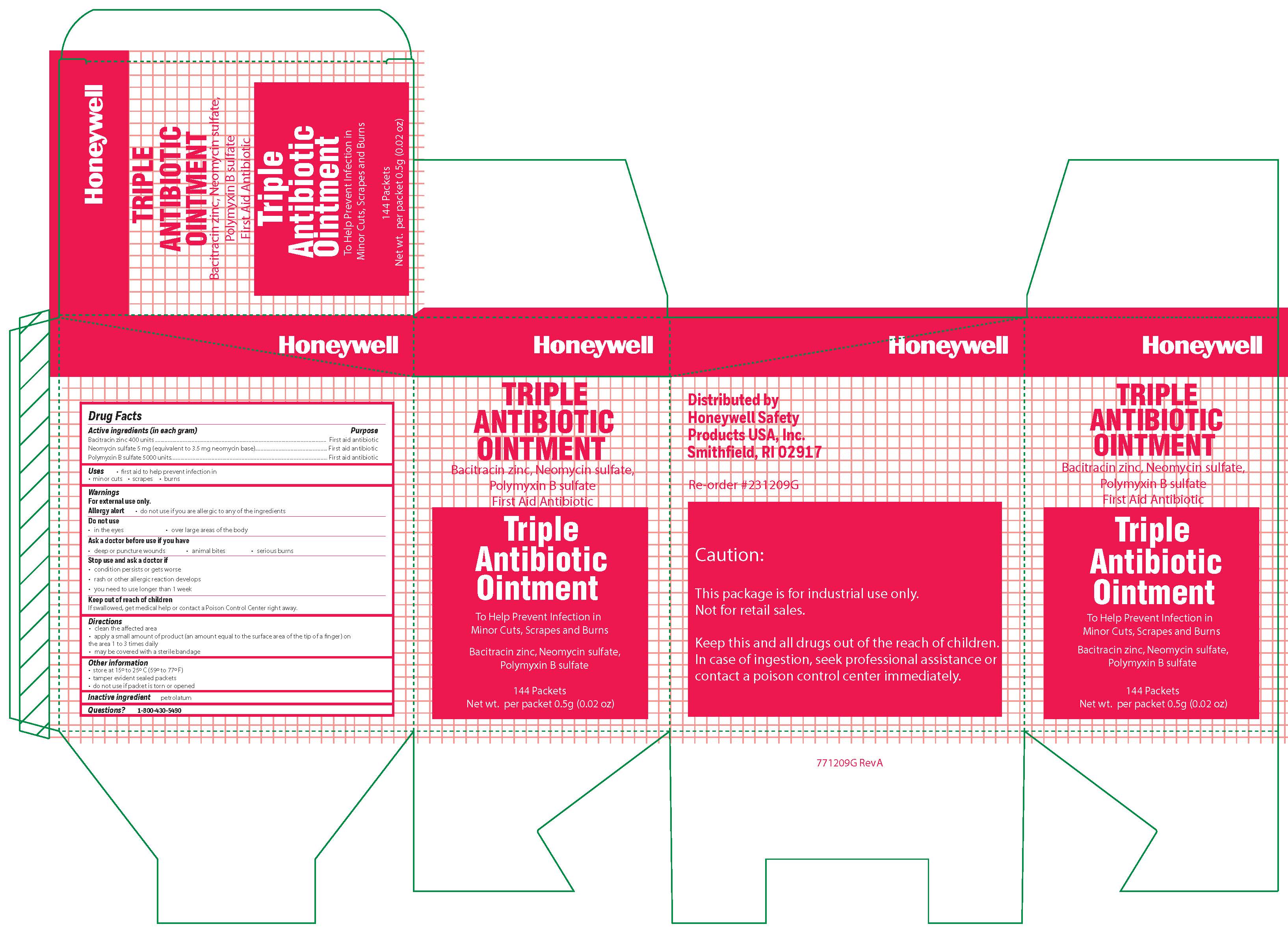
Triple
Active ingredient
Bacitracin zinc 400 units
Neomycin sulfate (5 mg equivalent to 3.5 mg Neomycin base)
Polymyxin B sulfate 5000 units
Triple
Warnings
For external use only
Allergy alert: do not use if you are allergic to any of the ingredients
Do not use
- in the eyes
- over large areas of the body
- Ask a doctor before use if you have
- a deep or puncture wounds
- animal bites
- serious burns
Triple
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Triple
Other information
- store at 15 0 to 25 0 C (59 0 to 77 0 F)
- tamper evident sealed packets
- do not use if packet is torn or opened
BZK Wipe
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
BZK Wipe
Other information
- store at room temperature 15 0 to 30 0 C (59 0 - 86 0 F)
- do not reuse towelette
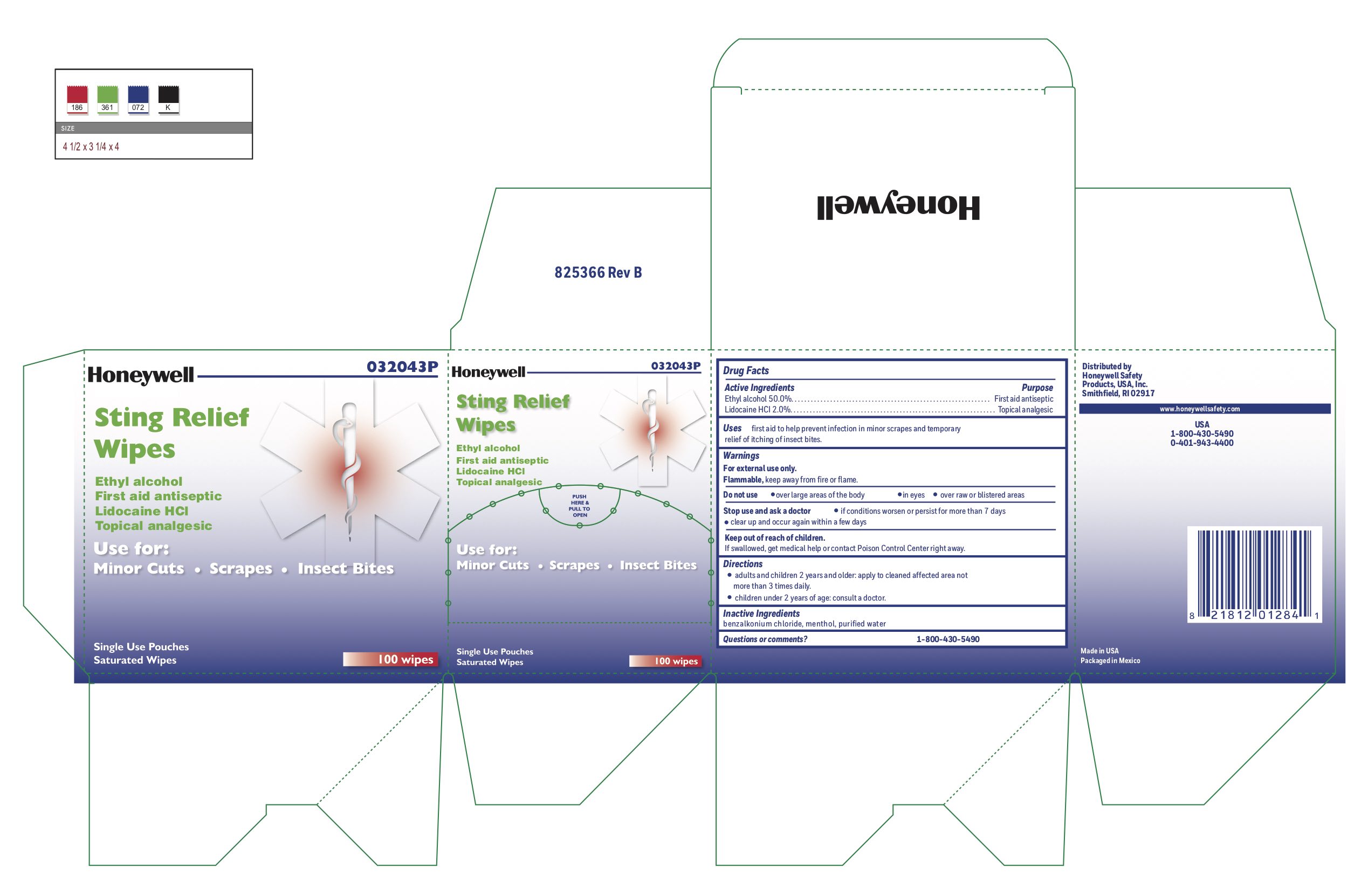
Sting Relief
Uses
- prevent infection in minor scrapes, and temporary relief of itching of insect bites
Sting Relief
Warnings
For external use only
Flammable, keep away from open fire or flame
Sting Relief
Directions
- adults and children 2 years and older: Apply to cleaned affected area not more than 3 times daily.
- children under 2 years of age: consult a doctor.
Eyewash
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyewash
Warnings
For external use only
- Obtain immediate medical treatment for all open wounds in or near eyes.
- To avoid contamination, do not touch tip of container to any surface.
- Do not reuse.
- Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Eyewash
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
PAWS
Uses
- for handwashing to decrease bacteria on skin whenever soap and water is not readily available
PAWS
Directions
- wet hands and wrists thoroughly for 15 seconds and allow to air dry
- always reseal after use
- children under 6 years of age should be supervised when using this product
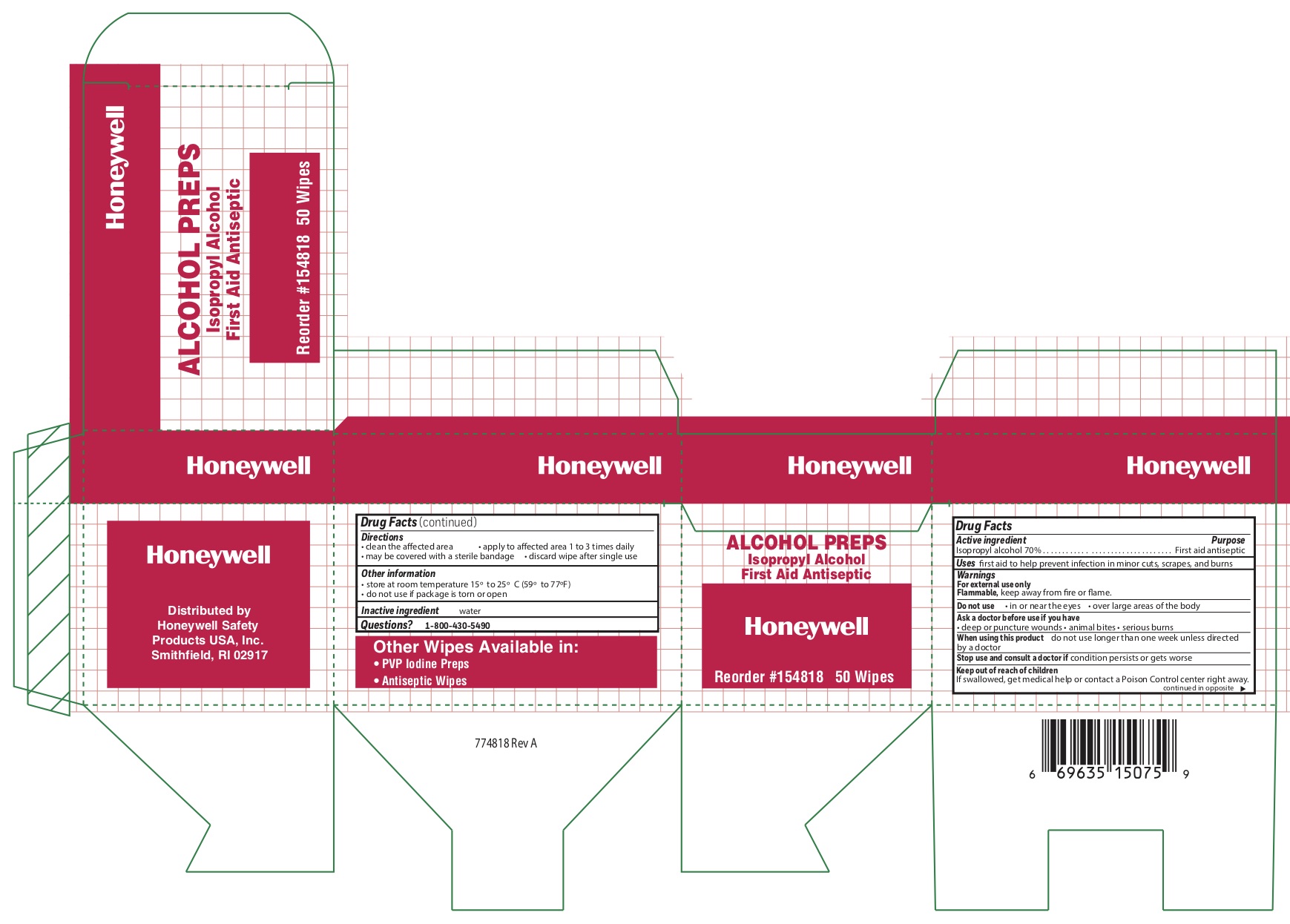
Alcohol
Directions
- clean the affected area
- may be covered with a sterile bandage
- apply wipe to affeted are 1 to 3 times daily
- discard wipe after single use
Alcohol
Other information
- store at room temperature 15 0 to 25 0 C (59 0 to 77 0 F)
- do not use if packet is torn or opened
4166
6836JEA Kit Contents
1 FINGERTIP 8 WOVEN 25/BOX
1 1X3 WOVEN SING 50/BOX
1 SWIFT KNUCKLE 40/BX
1 TRIPLE ANTIBIOTIC 10 PER
1 INSTANT COLD PACK 4" X 6"
1 BURN JEL 1/8 OZ, 6 PER
1 ALCOHOL PREP PADS 10P
1 ANTIMCRBL ANTSPTC TWLETTS
1 ADHESIVE TAPE W/P 1" X 10YDS
1 FIRST AID GUIDE ASHI
1 GAUZE CLEAN-WRAP BDGE N/S 2"
1 BLOODSTOPPER
1 GZE PADS STERILE 3"X 3" 10'S
1 CO-FLEX BANDAGE 2"X 5YDS TAN
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
LBL STOCK 6-3/8"X4"
1 LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 KIT STL 36 UN WHT 01 HOR SHELF
1 TRI BNDG NON WOVEN 40"X40"X56"
1 STING RELIEF SWAB 10
1 CPR MSK,WPS,GLVS 1