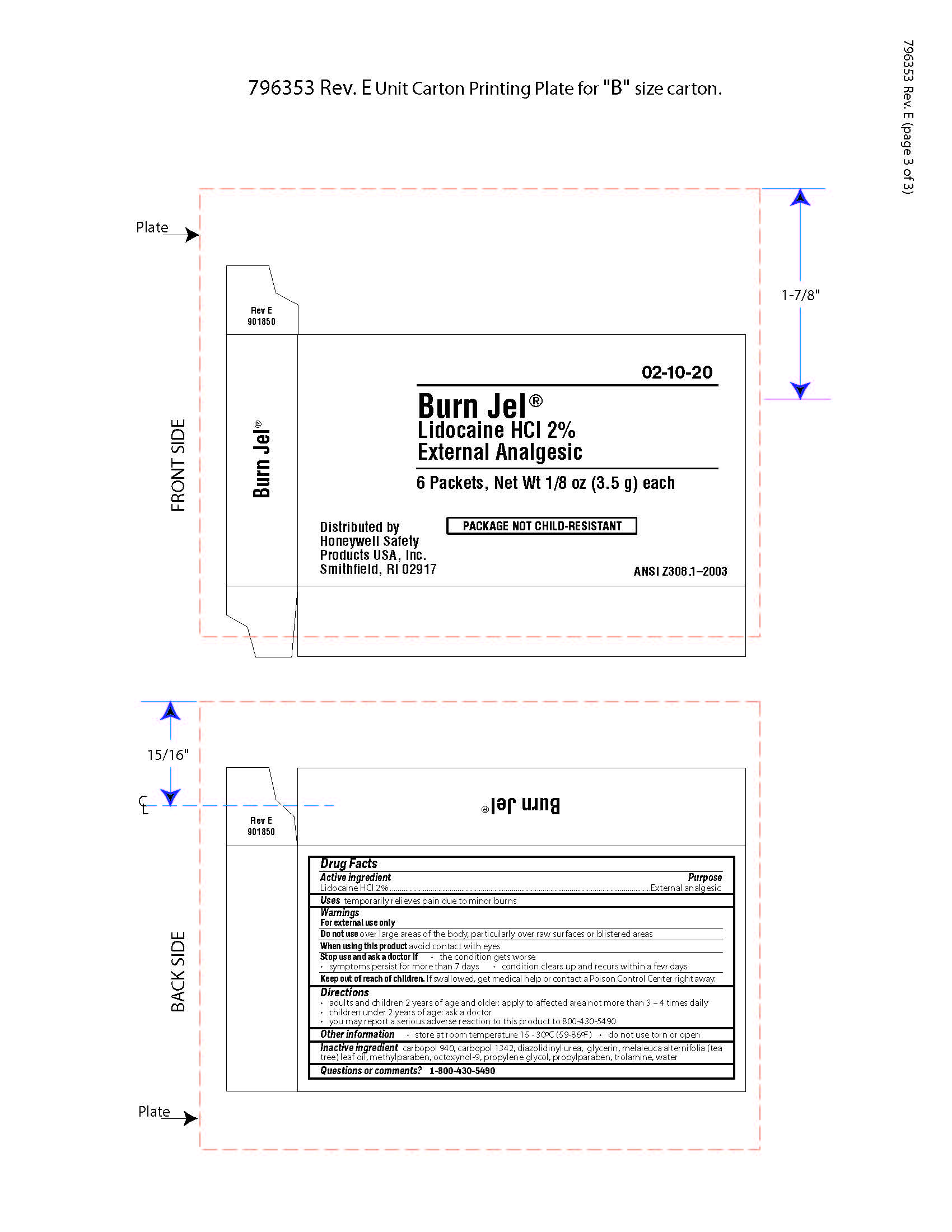
Burn Jel
Warnings
For external use only
Burn JEl
Directions
- adults and children 2 years of age and older; apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
- you may report a serious reaction to this product to 800-430-5490
Burn Jel
Inactive ingredients
carbopol 940, carbopol 1342, diazolidinyl urea, glycerin, melaleuca alternifolia (tea tree) leaf oil, methylparaben, octoxynol-9, propylene glycol, propylparaben, trolamine, water
BZK Wipe
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
BZK Wipe
Other information
- store at room temperature 15 0 to 30 0 C (59 0 - 86 0 F)
- do not reuse towelette
Eyewash
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyewash
Warnings
For external use only-
Obtain immediate medical treatment for all open wounds in or near eyes.
To avoid contamination, do not touch tip of container to any surface.
Do not reuse. Once opened, discard.
Do not use
if solution changes color or becomes cloudy
if you have open wounds in or near the eyes, get medical help right away.
Eyewash
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
4136
6824PIZ Kit Contents
1 BLUE WOVEN 1X3 100/BX 12/CS
2 INSTANT COLD PACK 4" X 6"
1 BURN JEL 1/8 OZ, 6 PER
1 ANTIMCRBL ANTSPTC TWLETTS
1 FIRST AID GUIDE ASHI
2 4OZ BFS EYEWASH TRILINGUAL BOTTLE
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
2 PR LRG NITRILE GLVES ZIP BAG
1 ANCHOR/SCREW 2 EACH ZIP BAG
1 SELF-ADH WRAP 2 X 5 YDS NORTH REV D
1 KIT STL 24 UN WHITE 01
1 GAUZE PADS 3"X3" 4/BX
4322
SF00004640 kit contents
1 BLUE WOVEN 1X3 100/BX 12/CS
2 INSTANT COLD PACK 4" X 6"
1 BURN JEL 1/8 OZ, 6 PER
1 ANTIMCRBL ANTSPTC TWLETTS
1 FIRST AID GUIDE ASHI
2 4OZ BFS EYEWASH TRILINGUAL BOTTLE
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
2 PR LRG NITRILE GLVES
1 ANCHOR/SCREW 2 EACH ZIP BAG
1 SELF-ADH WRAP 2 X 5 YDS NORTH REV D
1 KIT STL 24 UN WHITE 01
1 GAUZE PADS 3"X3" 4/BX