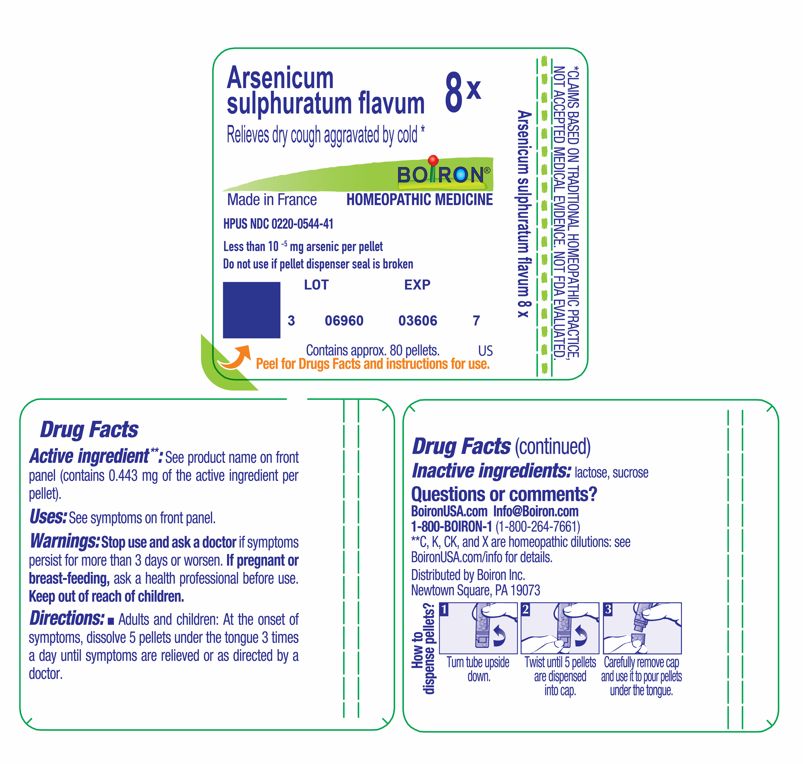
Arsenicum sulphuratum flavum 8X
Less than 10 -5 mg arsenic per pellet
Active ingredient**: See product name on front panel (**contains 0.443 mg of the active ingredient per pellet).
Adults and children: At the onset of symptoms, dissolve 5 pellets under the tongue 3 times a day until symptoms are relieved or as directed by a doctor.
Do not use if pellet dispenser seal is broken.
Contains approx 80 pellets.
How to dispense pellets? Turn tube upside down. Twist until 5 pellets are dispensed into cap. Carefully remove the cap and use it to pour pellets under the tongue.
*CLAIMS BASED ON TRADITIONAL HOMEOPATHIC PRACTICE NOT ACCEPTED MEDICAL EVIDENCE. NOT FDA EVALUATED.
*C,K,CK, and X are homeopathic dilutions: see BoironUSA.com/info for details.