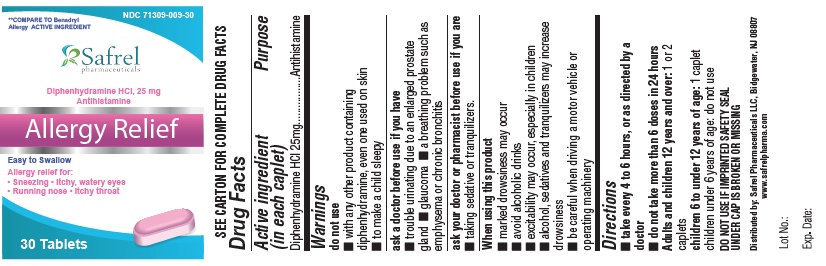
Uses
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itchy nose or throat
- temporarily relieves these symptoms of the common cold:
- runny nose
- sneezing
Warnings
Do not use
- with any other product containing diphenhydramine, even one used on skin
- to make a child sleepy
Ask a doctor before use if you have
- trouble urinating due to an enlarged prostate gland
- glaucoma ? a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if youare taking sedatives or tranquilizers
When using this product
- marked drowsiness may occur
- avoid alcoholic drinks
- excitability may occur, especially in children ? alcohol, sedatives and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
Keep out of reach of children.
In case of accidental overdose, contact a doctor or Poison Control Center immediately. Prompt medical attention is critical for adults as well as for children even if you do not notice any signs or symptoms.
Do not exceed recommended dosage.
Directions
- take every 4 to 6 hours, not more than 6 doses in 24 hours
- Adults and children 12 years of age and older: 1 or 2 tablets
- children 6 to under 12 years of age: 1 tablet
- children 4 to under 6 years of age: do not use unless directed by a doctor
- children under 4 years of age: do not use
Other Information
- each tablet contains : calcium 20 mg
- store at controlled room temperature 20°-25°C (68°-77°F).
- read all product information before using.
- TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING.
Inactive Ingredients
Colloidal silicon Dioxide, Croscarmellose Sodium, Dicalcium Phosphate, D & C Red, Magnesium stearate, Microcrystalline cellulose, Polyvinyl alcohol, Titanium dioxide, Talc
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
DIPHENHYDRAMINE HYDROCHLORIDE TABLET
, USP 25 MG
ANTIHISTAMINE
* This product is not manufactured or distributed by McNeil-Consumer Healthcare, owner of the registered trademark Benadryl� Allergy.
71309-109-10 100 Caplets
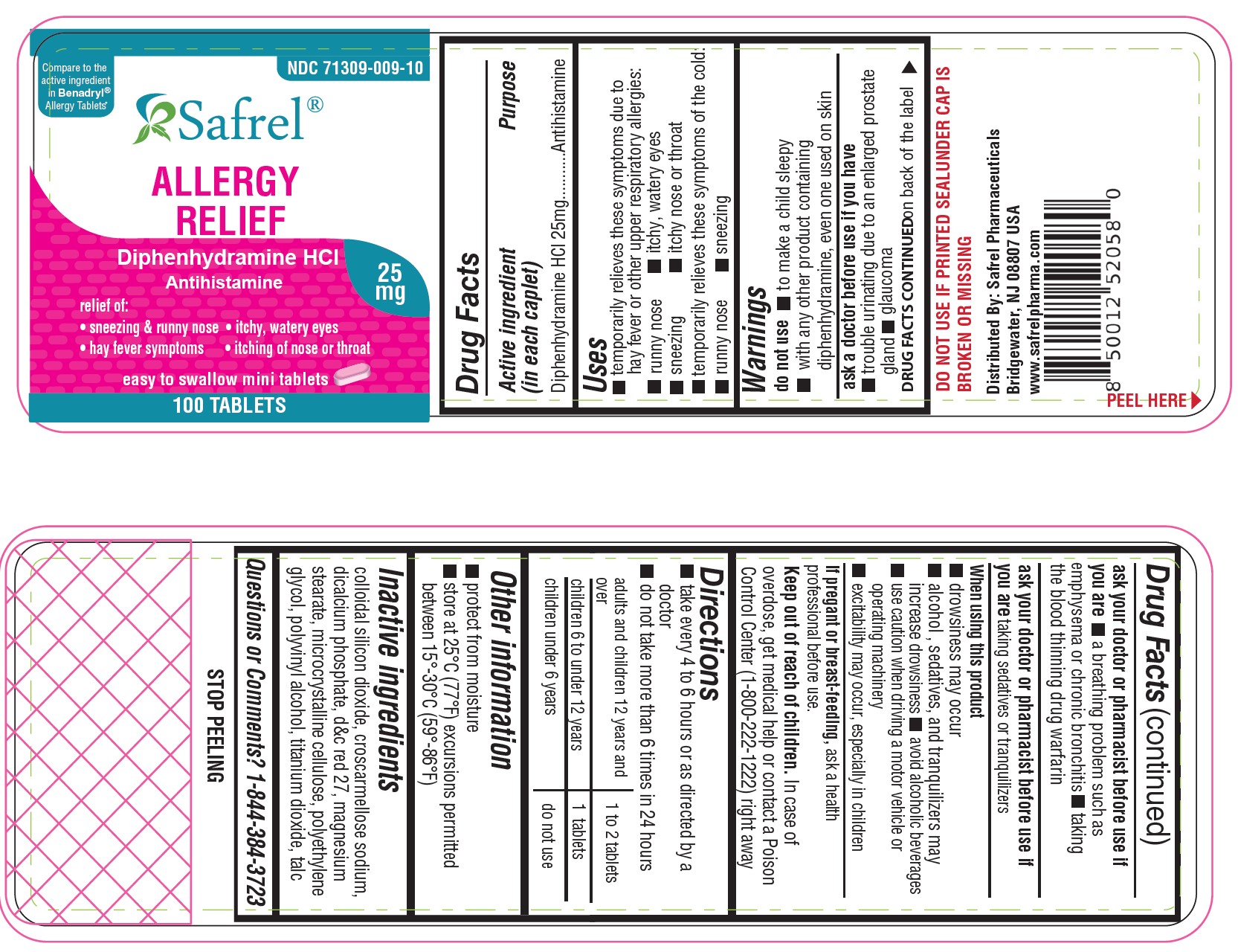
71309-109-06 600 Caplets
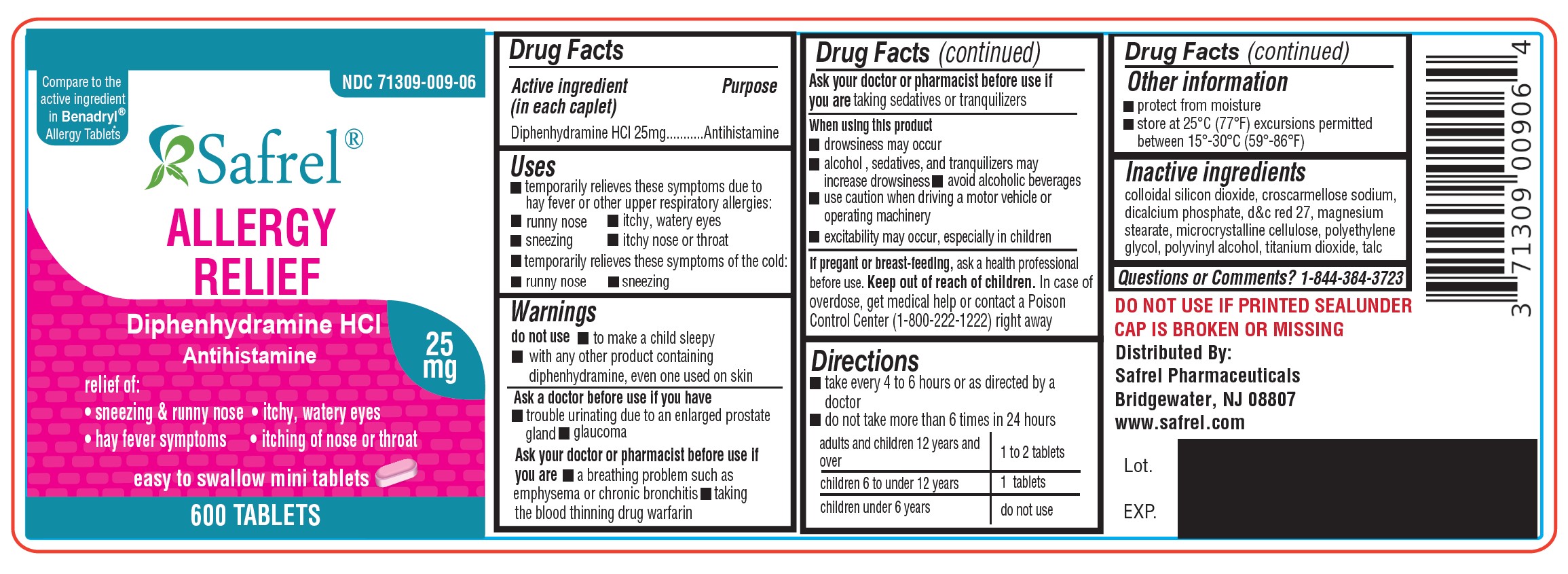
71309-109-01 1000 Caplets