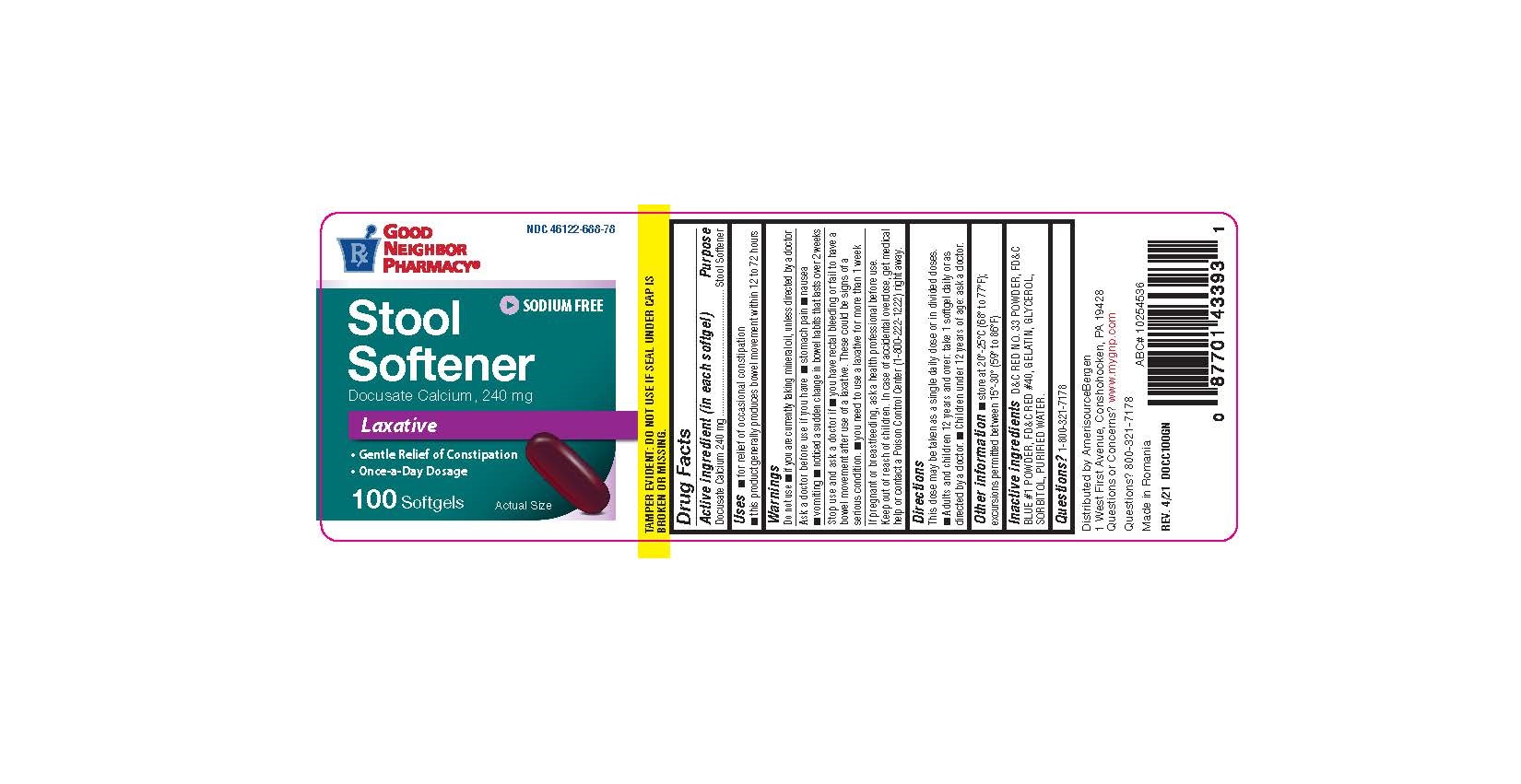
Uses
- For the relief of occasional constipation.
- This product generally produces a bowel movement within 12 to 72 hours.
Warnings: Do not use
- If you are currently taking mineral oil, unless directed by a doctor.
- When abdominal pain, nausea or vomiting are present.
- For longer than one week unless directed by a doctor.
Ask a Doctor Before Use
■ stomach pain ■ nausea ■ vomiting ■ noticed a sudden change in bowel habits that lasts over 2 weeks
Keep Out of Reach of Children.
In case of accidental overdose, get medical help or contact a Poison Control Center (1-800-222-1222) right away.
Directions
Adults and Children over 12 years of age
Take orally 1 softgel daily for 2 to 3 days or until bowl movements are normal, or as directed by a doctor.
Children under 12 years of age
Do not use this product for children under 12 years of age, unless directed by a doctor.
Other Information
- Store at room temperature between 15ºC to 25ºC (59ºF to 77ºF).
- Do not use if either seal is broken or missing.