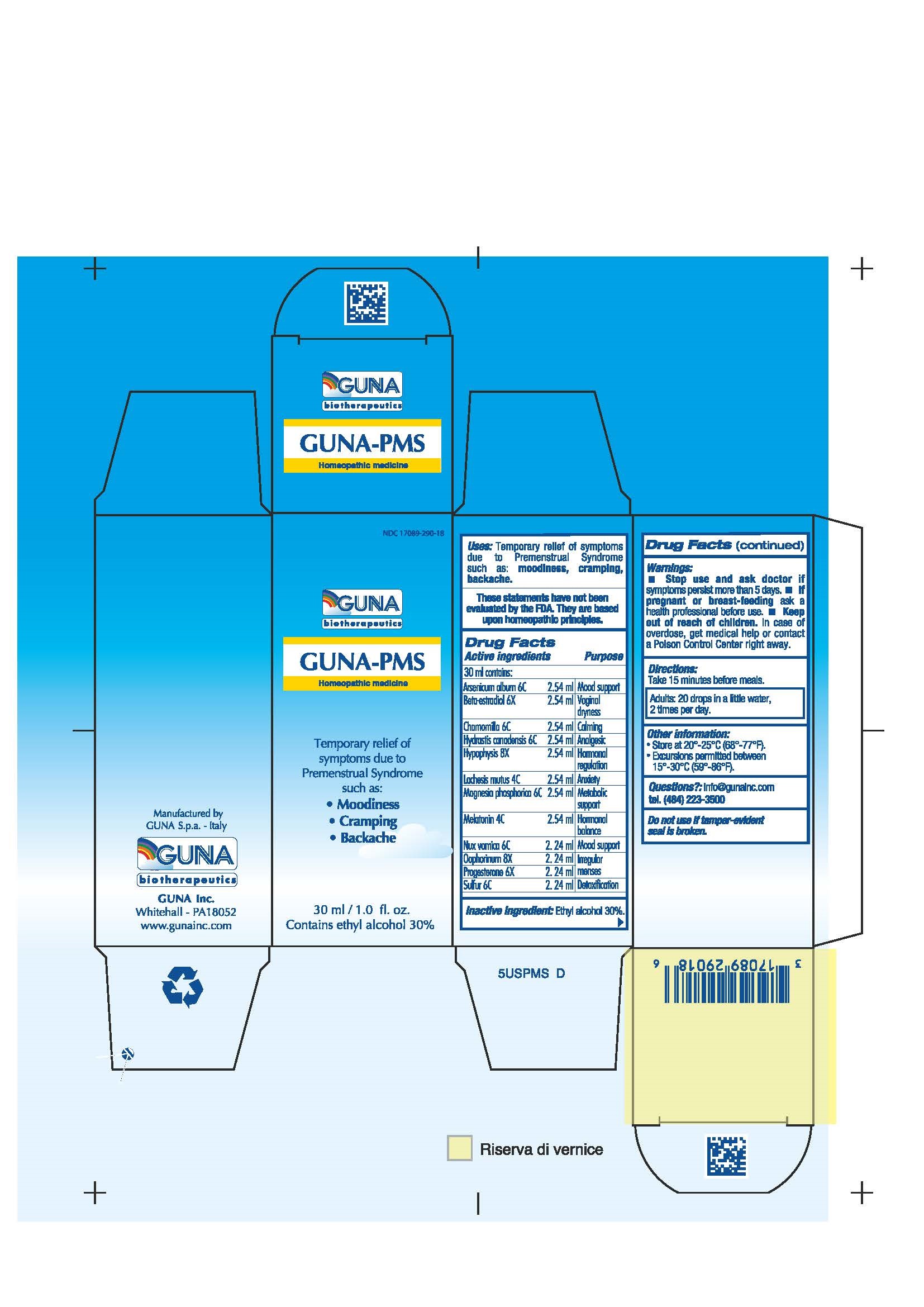
GUNA-PMS- arsenic trioxide - estradiol - goldenseal - lachesis muta venom - magnesium phosphate - matricaria recutita - melatonin - progesterone - strychnos nux-vomica seed - sulfur - sus scrofa ovary - sus scrofa pituitary gland - solution/ drops
Guna spa
Disclaimer: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
----------
ACTIVE INGREDIENTS/PURPOSE
ARSENICUM ALBUM 6C MOOD SUPPORT
BETA-ESTRADIOL 6X VAGINAL DRYNESS
CHAMOMILLA 6C CALMING
HYDRASTIS CANADENSIS 6C ANALGESIC
HYPOPHYSIS 8X HORMONAL REGULATION
LACHESIS MUTUS 4C ANXIETY
MAGNESIA PHOSPHORICA 6C METABOLIC SUPPORT
MELATONIN 4C HORMONAL BALANCE
NUX VOMICA 6C MOOD SUPPORT
OOPHORINUM 8X IRREGULAR MENSES
PROGESTERONE 6X IRREGULAR MENSES
SULFUR 6C DETOXIFICATION
USES
Temporary relief of symptoms due to Premenstrual Syndrome such as:
-
Moodiness
-
Cramping
-
Backache
PREGNANCY
If pregnant or breast-feeding ask a doctor before use
WARNINGS
Stop use and ask doctor if symptoms persist more than 5 days.
If pregnant or breast-feeding ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Contains ethyl alcohol 30%
DIRECTIONS
Adults: 20 drops in a little water, 2 times per day.
QUESTIONS
Questions?: info@gunainc.com, tel. (484) 223-3500
KEEP OUT OF REACH OF CHILDREN
Take 15 minutes before meals
Inactive ingredient: Ethyal alcohol 30%.
PRINCIPAL DISPLAY PANEL