Eyewash
Active ingredient
Sterile Water 99%
Eyewash
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyewash
Warnings
For external use only Obtain immediate medical treatment for all open wounds in or near eyes. To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Stop use and ask a doctor if
- you experience eye pain
- changes in vision
- continued redness or irritation of the eye
- condition worsens or persists
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away.
Eyewash
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
Eyewash
Inactive ingredients
sodium chloride, sodium phosphate dibasic, sodium phosphate monobasic
Eyewash
Questions
1-800-430-5490
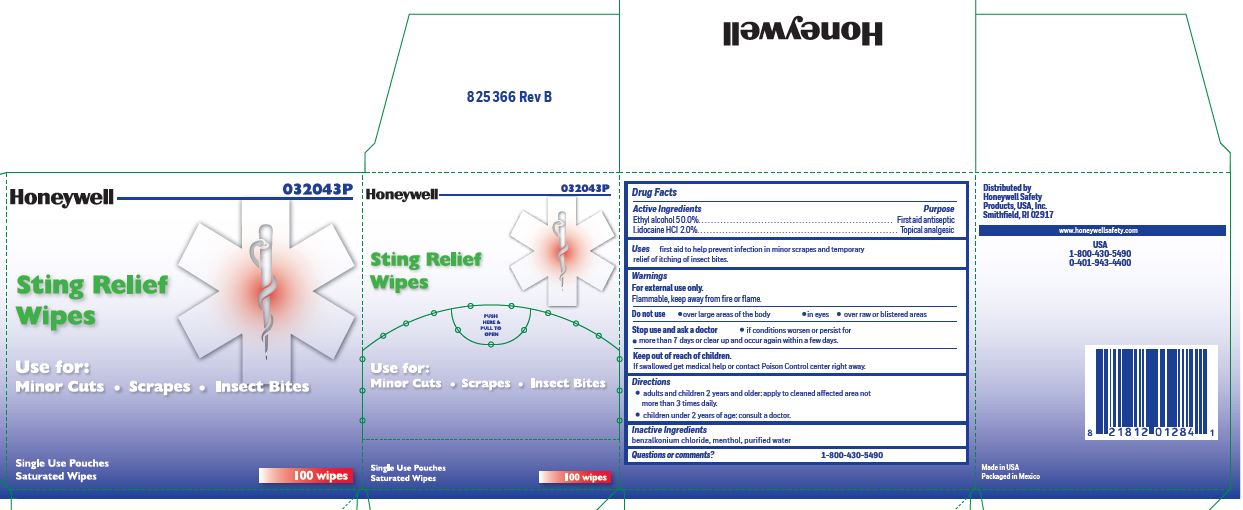
Sting Relief
Active ingredient (in each wipe)
Ethyl alcohol 50.0% Lidocaine HCl 2.0%
Sting Relief
Purpose
Antiseptic-Topical pain relief
Sting Relief
Uses
- prevent infection in minor scrapes, and temporary relief of itching of insect bites
Sting Relief
Warnings
For external use only
Flammable, keep away from open fire or flame
Do not use
- over large areas of the body
- in eyes
- over raw or blistered areas
Stop use and ask a doctor
- if conditions worsen or persist for more than 7 days or clear up and occur again within a few days
Keep out of reach of children.
- If swallowed, get medical help or contact a Poison Control Center right away.
Sting Relief
Directions
- adults and children 2 years and older: Apply to cleaned affected area not more than 3 times daily.
- children under 2 years of age: consult a doctor.
Sting Relief
Inactive ingredients
benzalkonium chloride, menthol, and purified water
Questions or Comments?
1-800-430-5490
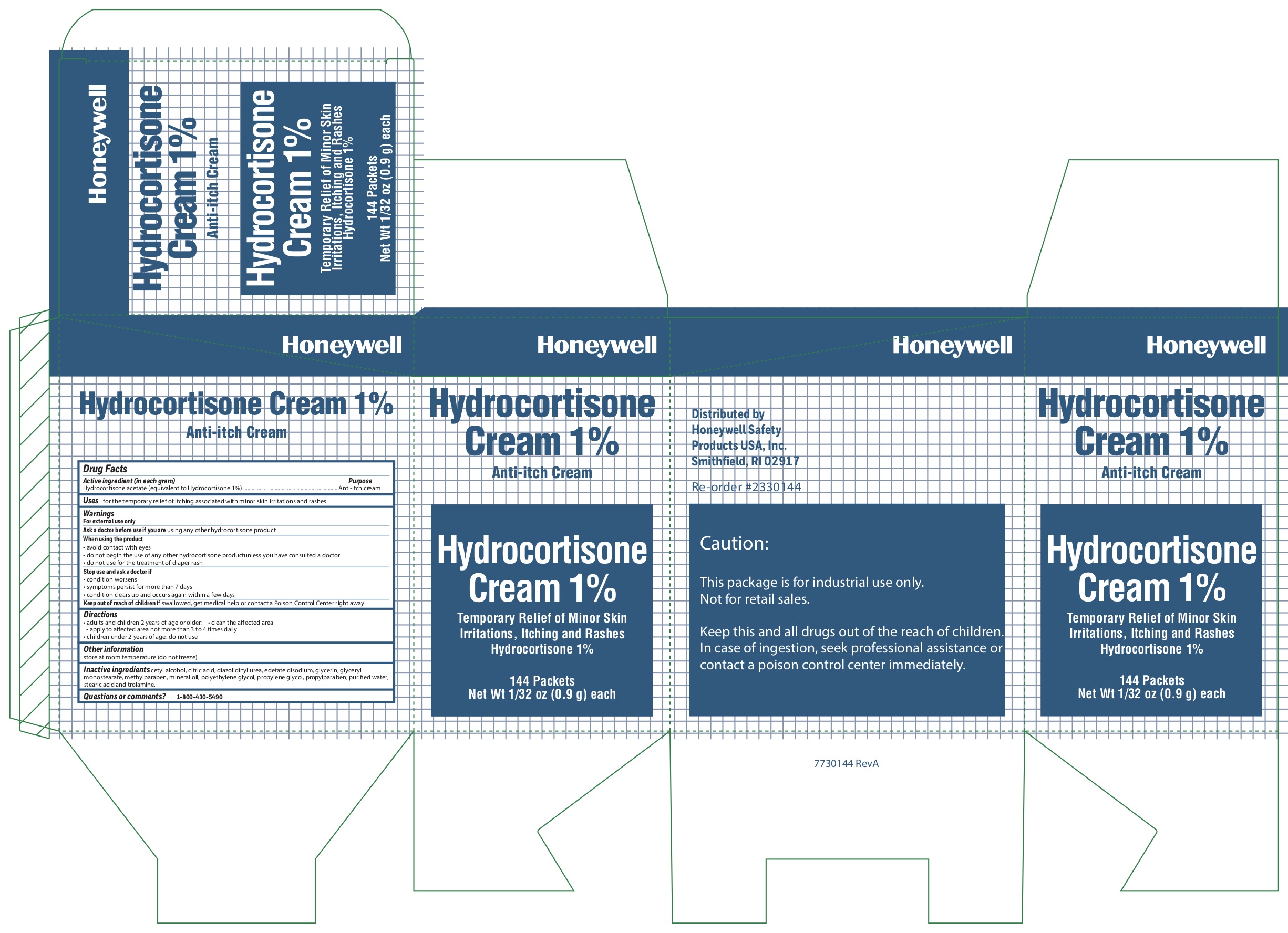
Hydrocortisone Cream
Active ingredient (in each gram)
Hydrocortisone acetate (equivalent to Hydrocortisone 1%)
Hydrocortisone
Purpose
Anti-itch cream
Hydrocortisone
Uses
- for the temporary relief of itching associated with minor skin irritations and rashes
Hysrocortisone
Warnings
For external use only
Ask a doctor before use if
- you are using any other hydrocortisone product
When using the product
- avoid contact with eyes
- do not begin use of any other hydrocortisone product unless you have consulted a doctor
- do not use for the treatment of diaper rash
Stop use and ask a doctor if
- condition worsens
- condition persists for more than 7 days
- condition clears up and recurs within a few days
Keep out of reach of children
- If swallowed, get medical help or contact a Poison Control Center right away.
Hydrocortisone
Directions
-
adults and children 2 years and older:
- clean the affected area
- apply to the area not more than 3 to 4 times daily
-
children under 2 years of age: consult a doctor
Hydrocortisone
Other information
- store at room temperature (do not freeze)
Hydrocortisone
Inactive ingredients
cetyl alcohol, citric acid, diazolidinyl urea, edetate disodium, glycerin, glyceryl monostearate, methylparaben, mineral oil, polyethylene glycol, propylene glycol, propylparaben ..
Hydrocortisone
Questions or Comments?
1-800-430-5490
PVP
Active ingredient
Povidone-iodine 10%
(equivalent to 1% titratable iodine)
PVP
Purpose
First aid antiseptic
PVP
Uses
- first aid antiseptic to help prevent infection in minor cuts, scrapes and burns
PVP
Warnings
For external use only.
Ask a doctor before use if you have
- deep or puncture wounds
- animal bites
- serious burns
Stop use and ask a doctor if
- condition worsens or persists for more than 72 hours
- irritation and redness develops
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
PVP
Directions
- clean the affected area
- apply1 to 3 times daily
- may be covered with a sterile bandage
- if bandaged, let dry first
- discard wipe after single use
PVP
Other information
- do not use on individuals who are allergic or sensitive to iodine
- store at controlled temperature 59-86ºF (15-30ºC)
- do not use if pouch is open or torn
PVP
Inactive ingredients
nonoxynol 9, water
PVP
Questions
1-800-430-5490
Ammonia
Active ingredient
Ammonia 15%
Ammonia
Purpose
Respiratory stimulant
Ammonia
Uses
- to prevent or treat fainting
Ammonia
Warnings
For external use only
Do not use
- if you have breathing problems such as asthma or emphysema
Stop use and ask a doctor if
Keep out of reach of children
- If swallowed get medical help or contact a Poison Control Center right away.
Ammonia
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Ammonia
Other information
- store at room temperature away from light
Ammonia
Inactive ingredients
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
Ammonia
Questions
1-800-430-5490
4286
SF00002863 Kit Contents
1 KNUCKLE BAND 8 PER
1 AMMONIA INHALANTS 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
2 TRIANGULAR BDG, NON-STERILE
1 WIRE SPLINT 1 PER
1 ADH TAPE, .5" X 2.5 YD, 2 PER
1 GAUZE COMPRESS, 1728 SQ IN 1
2 GAUZE BANDAGE, 2" X 6 YD,2 PER
1 GAUZE COMP, 18" X 36", 2 PER
1 INSTANT COLD PACK 4" X 6"
1 BUFFERED EYE WASH 1 OZ BTL
1 FINGERTIP BANDAGE, 10 PER
1 WATER JEL DRESSING 4" X 4"
1 HYDROCORTISON,1.O%,1/32 OZ,10P
1 PVP IODINE WIPES 10 PER
1 STING RELIEF WIPES 10 PER BOX
1 IVYX CLEANSER TOWEL 5 PER
1 FACE MASK W/SHIELD 1 PER BBP
1 NITRILE GLOVES 2PR BBP
1 ADH BDG, CLOTH, 1"X3", 16 PER
1 CPR MICROSHIELD DOUBLE UNIT
1 SPLINTER FORCEP 4 1/2"
1 SCISSOR LISTER BDG S/S 5 1/2"
1 BANDAGE COMP 2" W/TELFA PAD 4
1 BANDAGE COMP 4" W/TELFA PAD 1
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 KIT STL 36 UN WHT 01 HOR SHELF
Eyewash
Principal Display Panel

Sting Relief
Principal Display Panel
Hydrocortisone
Principal Display Panel
PVP
Principal Display Panel

Ammonia
Principal Display Panel

4286 Kit Label
SF00002863






