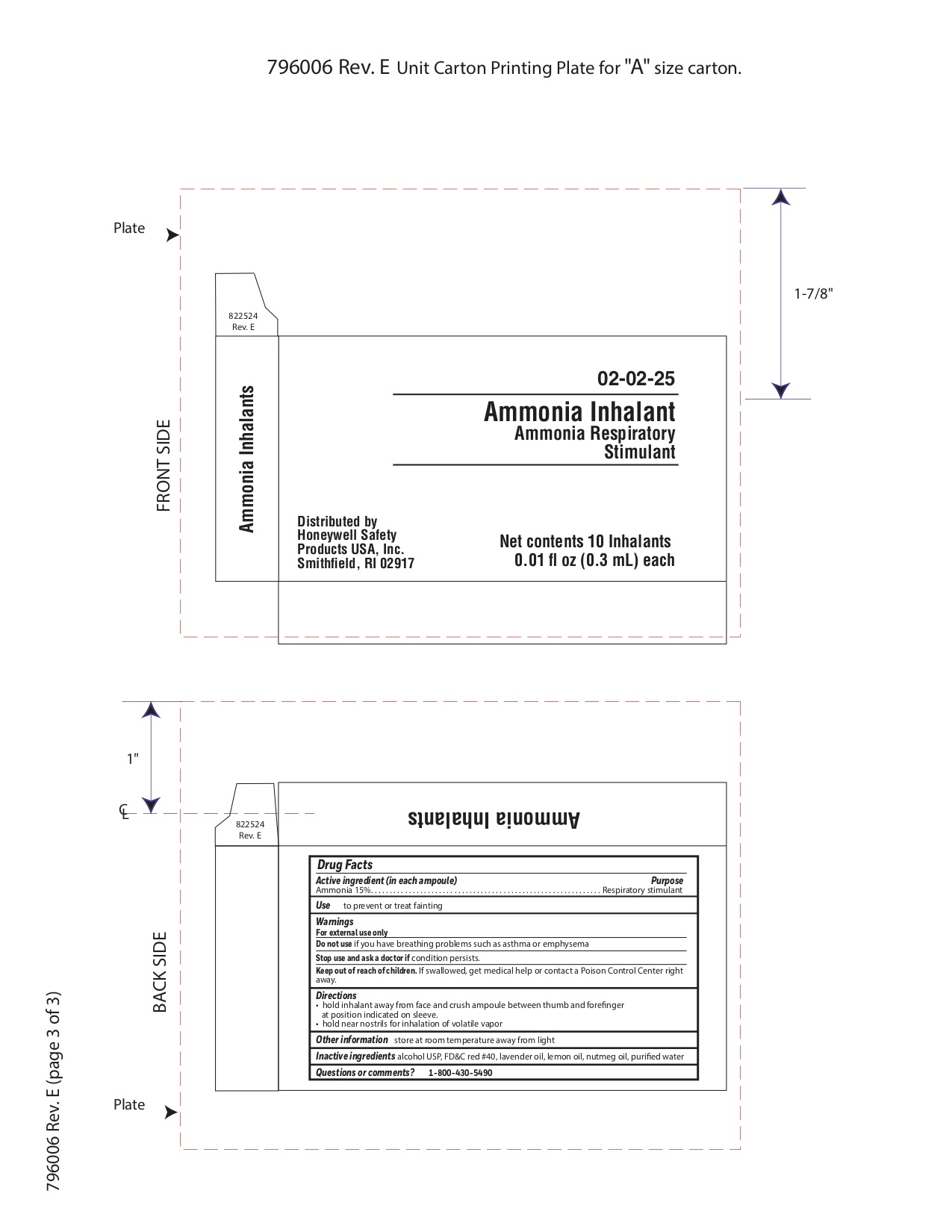
Ammonia Inhalent
Directions
- hold inhalant away from face and crush ampoule between thumb and forefinger at position indicated on sleeve.
- hold near nostrils for inhalation of volatile vapor
Ammonia Inhalent
Inactive ingredients
alcohol USP, FD&C red #40, lavender oil, lemon oil fcc, nutmeg oil, purified water
PVP
Warnings
For external use only
Keep out of reach of children.
- If swallowed, get medical help or contact a Poison Control Center right away
PVP
Directions
Reverse cardboard sleeve, then crush at dot between thumb and forefinger. Allow solution to saturate tip and apply solution to injury.
- clean affected area
- apply to affected area 1 to 3 times daily
- may be covered with a sterile bandage
- discard swab after single use
PVP
Other informatiion
- store at room temperature away from light
- keep from freezing or excessive heat
- do not use if package is torn or open
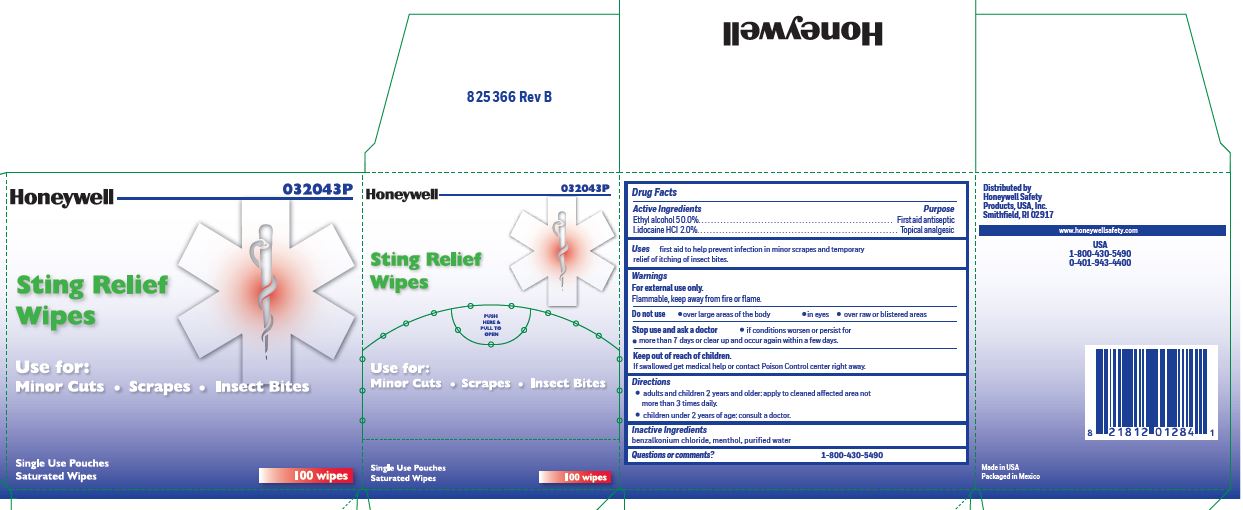
Sting Relief
Uses
- prevent infection in minor scrapes, and temporary relief of itching of insect bites
Sting Relief
Warnings
For external use only
Flammable, keep away from open fire or flame
Stig Relief
Directions
adults and children 2 years and older: Apply to cleaned affected area not more than 3 times daily.
children under 2 years of age: consult a doctor.
4270
SF00001090 Kit Contents
1 1 X 3 PLASTIC 50/BOX
1 WOVEN 2" X 3" 25/BOX
1 KNUCKLE BAND 8 PER
1 AMMONIA INHALANTS 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
2 INSTANT COLD PACK 4" X 6"
1 FINGERTIP BANDAGE, 10 PER
1 PVP IODINE WIPES 10 PER
2 ADHES TAPE W/P 1"X 2 1/2 YD
2 GAUZE BANDAGE 2"X2 YDS STRETCH GZ
1 FIRST AID GUIDE ASHI
1 GZE PADS STERILE 3"X 3" 10'S
1 CPR FILTERSHIELD 77-100
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
1 180 EMPTY BLANK NO LOGO
1 POCKET INSERT RED #180 KIT 4R
2 BANDAGE COMP 4" W/TELFA PAD 1
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
3 PR LRG NITRILE GLVES ZIP BAG
1 WATER-JEL BURN DRESSING 4 X 4
1 NOX A STING WIPES 10
4401
SF00001103 kit contents
1 1 X 3 PLASTIC 50/BOX
1 WOVEN 2" X 3" 25/BOX
1 KNUCKLE BAND 8 PER
1 AMMONIA INHALANTS 10 PER
1 EYE DRESS PKT W/4 ADH STRIPS
2 INSTANT COLD PACK 4" X 6"
1 FINGERTIP BANDAGE, 10 PER
1 PVP IODINE WIPES 10 PER
2 ADHES TAPE W/P 1"X 2 1/2 YD
2 GAUZE BANDAGE 2"X2 YDS STRETCH GZ
1 FIRST AID GUIDE ASHI
1 GZE PADS STERILE 3"X 3" 10'S
1 CPR FILTERSHIELD 77-100
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
2 BANDAGE COMP 4" W/TELFA PAD 1
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
3 PR LRG NITRILE GLVES ZIP BAG
1 WATER-JEL BURN DRESSING 4 X 4
1 POLYBAG 14" X 20" '3 MIL'
1 STING relief WIPES 10