Burn Jel
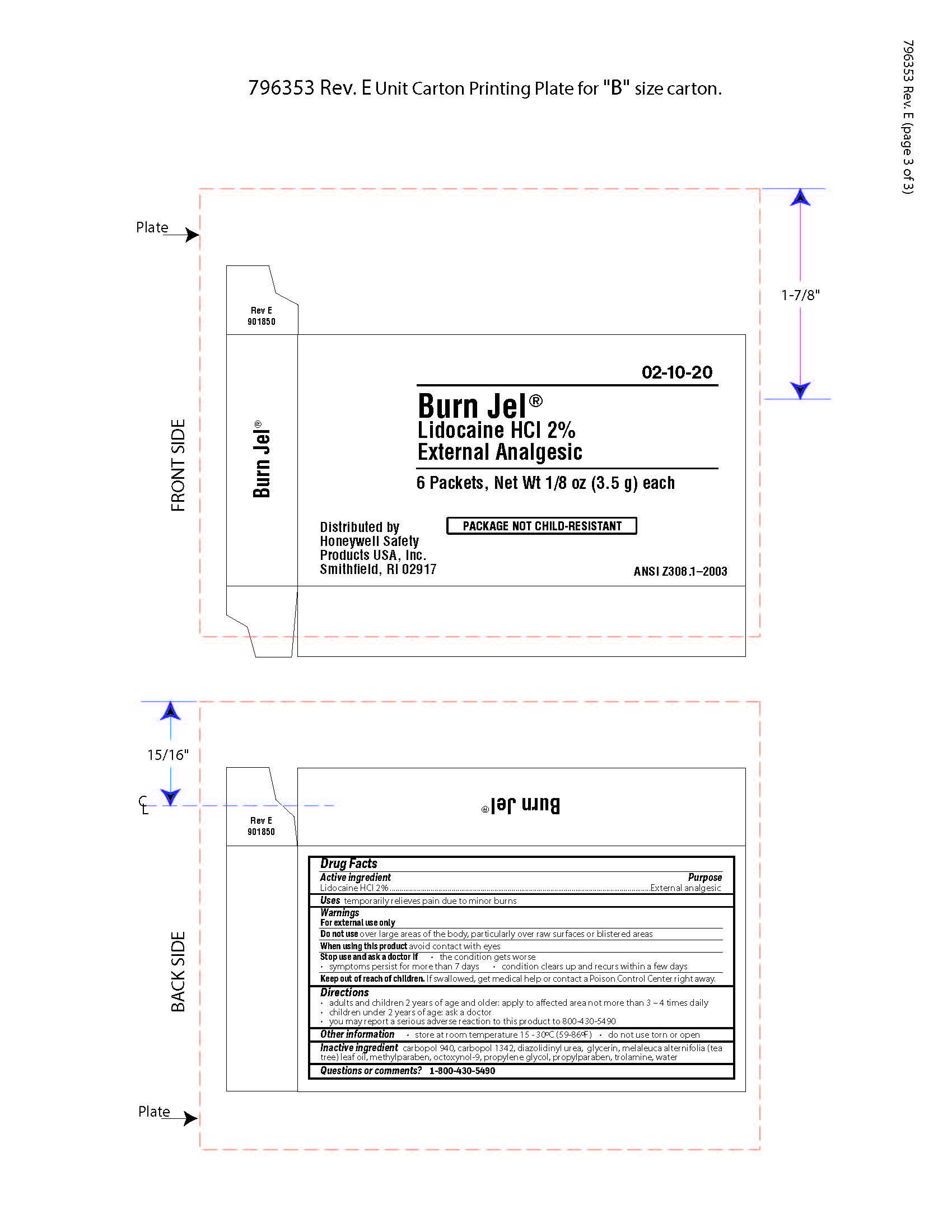
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Burn Jel
Directions
- adults and children 2 years of age and older; apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
- you may report a serious reaction to this product to 800-430-5490
Burn Jel
Inactive ingredients
carbopol 940, carbopol 1342, diazolidinyl urea, glycerin, melaleuca alternifolia (tea tree) leaf oil, methylparaben, octoxynol-9, propylene glycol, propylparaben, trolamine, water
Paws
Directions
- wet hands and wrists thoroughly for 15 seconds and allow to air dry
- always reseal after use
- children under 6 years of age should be supervised when using this product
4007
34TP165 Kit Contents
1 1 X 3 PLASTIC 50/BOX
1 GAUZE BANDAGE, 4" X 6 YD
1 TRIANGULAR BDG, NON-STERILE
1 INSTANT COLD PACK 4" X 6"
1 BURN JEL 1/8 OZ, 6 PER
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 GAUZE CLEAN-WRAP BDGE N/S 2"
1 ABD COMBINE PAD 5" X 9"
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
1 F. A. INST CHART SM (INDIVIDUAL LBL)
1 CPR KIT-MASK, GLOVES, WIPES
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 BODY FLUID CLEAN UP KIT BAG
1 KIT PP 24 UNIT FA
4013
34T150 kit contents
1 1 X 3 PLASTIC 50/BOX
1 GAUZE BANDAGE, 4" X 6 YD
1 TRIANGULAR BDG, NON-STERILE
1 INSTANT COLD PACK 4" X 6"
1 BURN JEL 1/8 OZ, 6 PER
1 ADHESIVE TAPE W/P 1/2"X 5 YD
1 GAUZE CLEAN-WRAP BDGE N/S 2"
1 ABD COMBINE PAD 5" X 9"
1 SCISSOR BDGE 4" RED PLS HDL
1 KIT TWEEZER 3 1/2" SLANTED
1 F. A. INST CHART SM (INDIVIDUAL LBL)
1 CPR KIT-MASK, GLOVES, WIPES
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 BODY FLUID CLEAN UP KIT BAG
1 KIT STL BULK MEDIUM