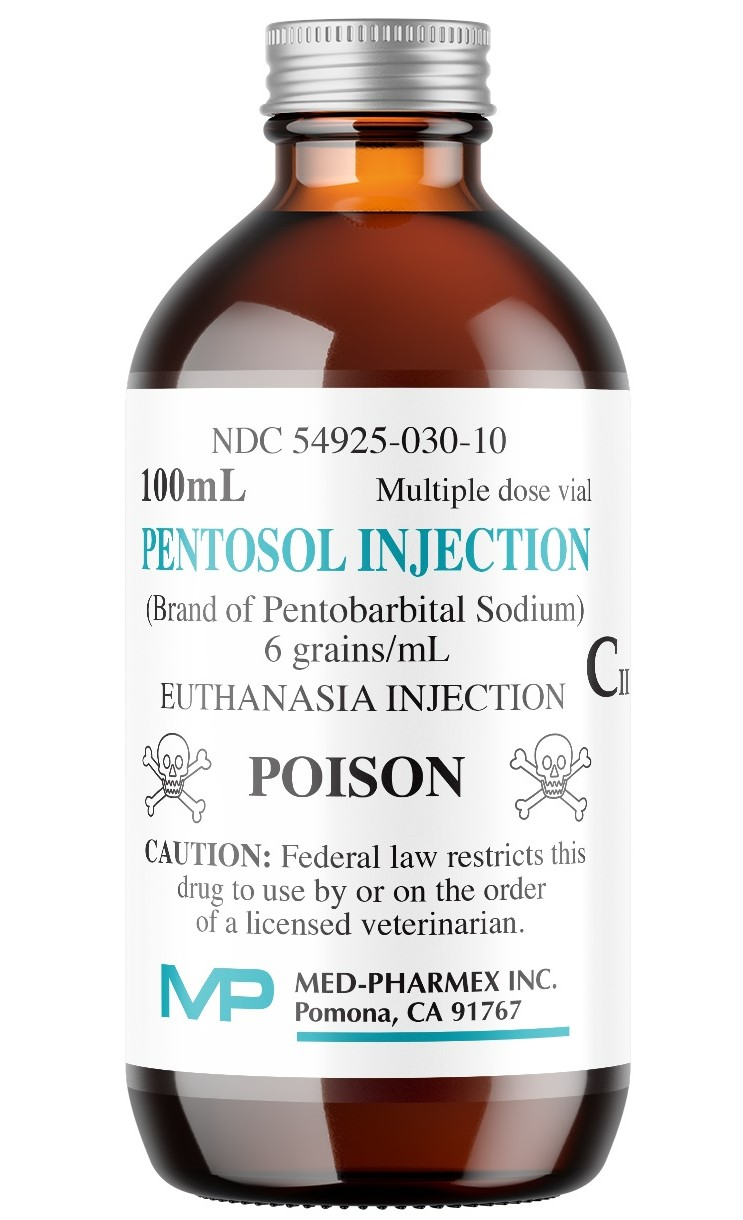
DESCRIPTION:
Pentosol (euthanasia) Injection
NDC 549245-030-10
FOR VETERINARY EUTHANASIA ONLY. Keep Out of Reach of Children.
CAUTION: Federal law restricts this drug to use by or on the order of a licensed veterinarian.
6 grains of pentobarbital sodium
Net contents: 100 mL
DOSAGE AND ADMINISTRATION:
Mulitple Dose Vial
Dosage: For intravenous (preferred), intracardial, intrapleural, or intraperitoneal injection.
Small Animals: Inject rapidly 1 mL per 10 lbs. of bady weight. Minimum dose 1 mL.
Horses and other Large Animals: Inject rapidly 1 mL per 10 lbs. of obdy weight, to a maximum of 100 mL.
Each mL contains:
Pentobarbital sodium ..... 6 gr.
Isopropyl alcohol .......... 10%
Propylene glycol ........... 18%
Benzyl alcohol .............. 2.0%
Purified water ............... qs
Green dye to establish a distinctive color.
TAKE TIME - OBSERVE LABEL DIRECTIONS
WARNING:
This is a highly toxic parenteral solution specifically designed for rapid, painless, and humane euthanasia of animals.
ENVIRONMENTAL HAZARD:
This product is toxic to wildlife. Birds and mammals feeding on treated animals may be killed. Euthanized animals must be properly disposed of by deep burial, incineration, or other method in compliance with state and local laws to prevent consumption of carcass material by scavenging wildlife.
To report suspected adverse drug events, for technical assistance, or to obtain a copy of the Safety Data Sheet (SDS), contact Med-Pharmex at (909) 593-7875. For additional information about adverse drug experience reporting for animals, contact FDA at 1-888-FDA-VETS or online at http://www.fda.gov/reportanimalae.
Manufactured by:
Med-Pharmex, Inc.
2727 Thompson Creek Road
Pomona, CA 91767
Rev.: 01/2023