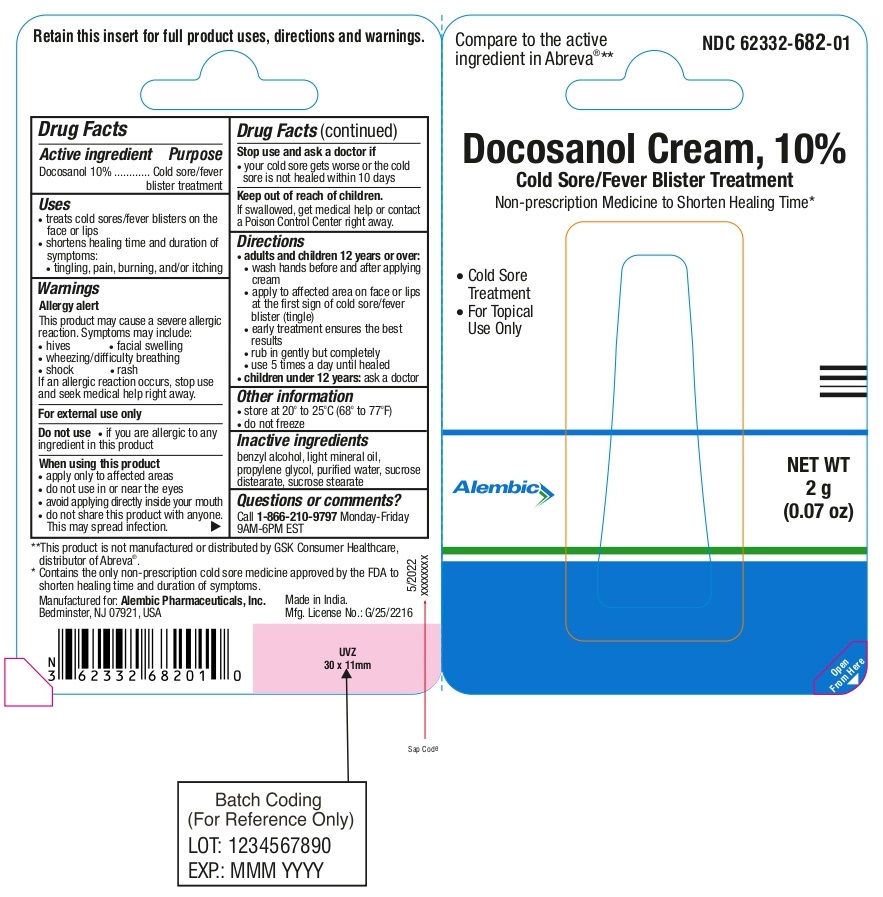
Uses
- treats cold sores/fever blisters on the face or lips
- shortens healing time and duration of symptoms:
Warnings
This product may cause a severe allergic reaction. Symptoms may include:
- hives
- facial swelling
- wheezing/difficulty breathing
- shock
- rash
If an allergic reaction occurs, stop use and seek medical help right away.
For external use only
Do not use
- if you are allergic to any ingredient in this product
- apply only to the affected areas
- do not use in or near the eyes
- avoid applying directly inside your mouth
- do not share this product with anyone. This may spread the infection.
- your cold sore gets worse or the cold sore is not healed within 10 days
Keep out of reach of children.
If swallowed, get medical help or contact a Poison Control Center right away.
Directions
- adults and children 12 years or over:
• wash hands before and after applying cream
• apply to affected area on face or lips at the first sign of cold sore/fever blister (tingle)
• early treatment ensures the best results
• rub in gently but completely
• use 5 times a day until healed
- children under 12 years:ask a doctor
Inactive ingredients
benzyl alcohol, light mineral oil, propylene glycol, purified water, sucrose distearate, sucrose stearate
Principal Display Panel
NDC 62332-682-01
Docosanol Cream, 10%
Compare to the active ingredient in Abreva®**
Cold Sore/Fever Blister Treatment
Non-prescription Medicine to Shorten Healing Time*
Cold Sore Treatment
For Topical Use Only
NET WT 2g (0.07 oz)
**This product is not manufactured or distributed by GSK Consumer Healthcare, distributor of Abreva®.
*Contains the only non-prescription cold sore medicine approved by the FDA to shorten healing time and duration of symptoms.
RETAIN THIS INSERT FOR FULL PRODUCT USES, DIRECTIONS AND WARNINGS
Manufactured for:
Alembic Pharmaceuticals, Inc.
Bedminster, NJ 07921, USA
Made in India.
NDC 62332-682-03
Docosanol Cream, 10%
Compare to the active ingredient in Abreva®**
Cold Sore/Fever Blister Treatment
Non-prescription Medicine to Shorten Healing Time*
Cold Sore Treatment
For Topical Use Only
NET WT 2g (0.07 oz)
**This product is not manufactured or distributed by GSK Consumer Healthcare, distributor of Abreva®.
*Contains the only non-prescription cold sore medicine approved by the FDA to shorten healing time and duration of symptoms.
RETAIN THIS INSERT FOR FULL PRODUCT USES, DIRECTIONS AND WARNINGS
Manufactured for:
Alembic Pharmaceuticals, Inc.
Bedminster, NJ 07921, USA
Made in India.
