Eyesaline
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyesaline
Warnings
For external use only-
Obtain immediate medical treatment for all open wounds in or near eyes.
To avoid contamination, do not touch tip of container to any surface.
Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Eyesaline
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
Eyesaline
Inactive ingredients
sodium chloride, sodium phosphate dibasic, sodium phosphate monobasic
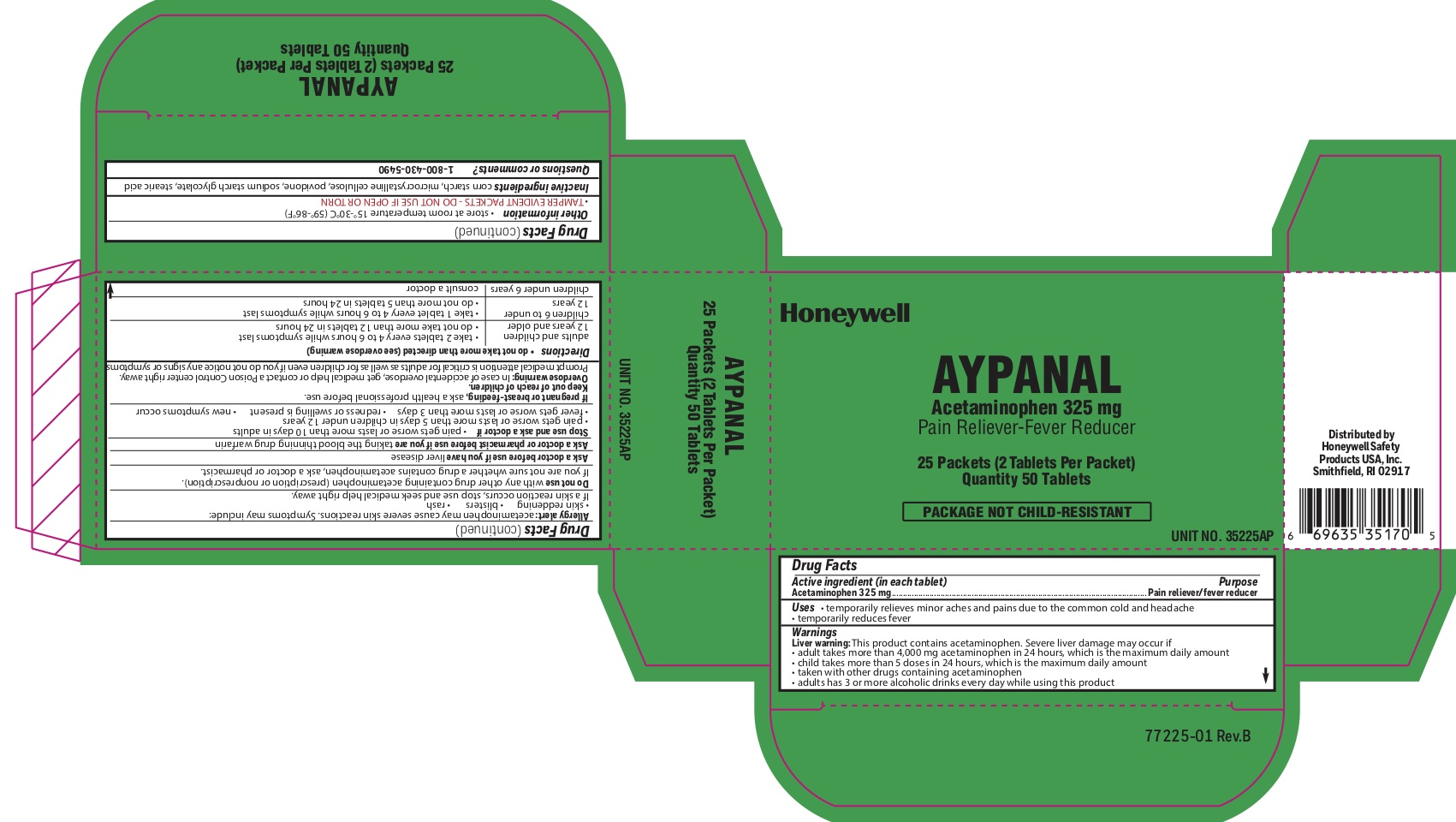
Aypanal
Uses
- temporarily relieves minor aches and pains due to the common cold and headache - temporarily reduces fever
Aypanal
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take:
- more than 4,000 mg in 24 hours, which is the maximum daily amount
- child takes more than 5 doses in 24 hours, which is the maximum daily amount
- taken with other drugs containing acetaminophen
- adult has 3 or more alcoholic drinks every day while using this product:
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
- If a skin rash occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription).
- If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
Aypanal
Directions
do not take more than directed (see overdose warning)
adults and children 12 years of age or older
- take two tablets every 4-6 hours while symptoms last
- do not take more than 12 tablets in 24 hours
children 6 to under 12 years of age
- take 1 tablet every 4-6 hours while symptoms last
- do not take more than 5 tablets in 24 hours
children under 6 years consult a doctor
Aypanal
Other information
- store at room temperature 15 0 to 30 0 C (59 0 - 86 0 F)
- TAMPER EVIDENT PACKETS- DO NOT USE IF OPEN OR TORN
Aypanal
Inactive ingredients
corn starch, microcrystalline cellulose, povidone, sodium starch glycolate, stearic acid
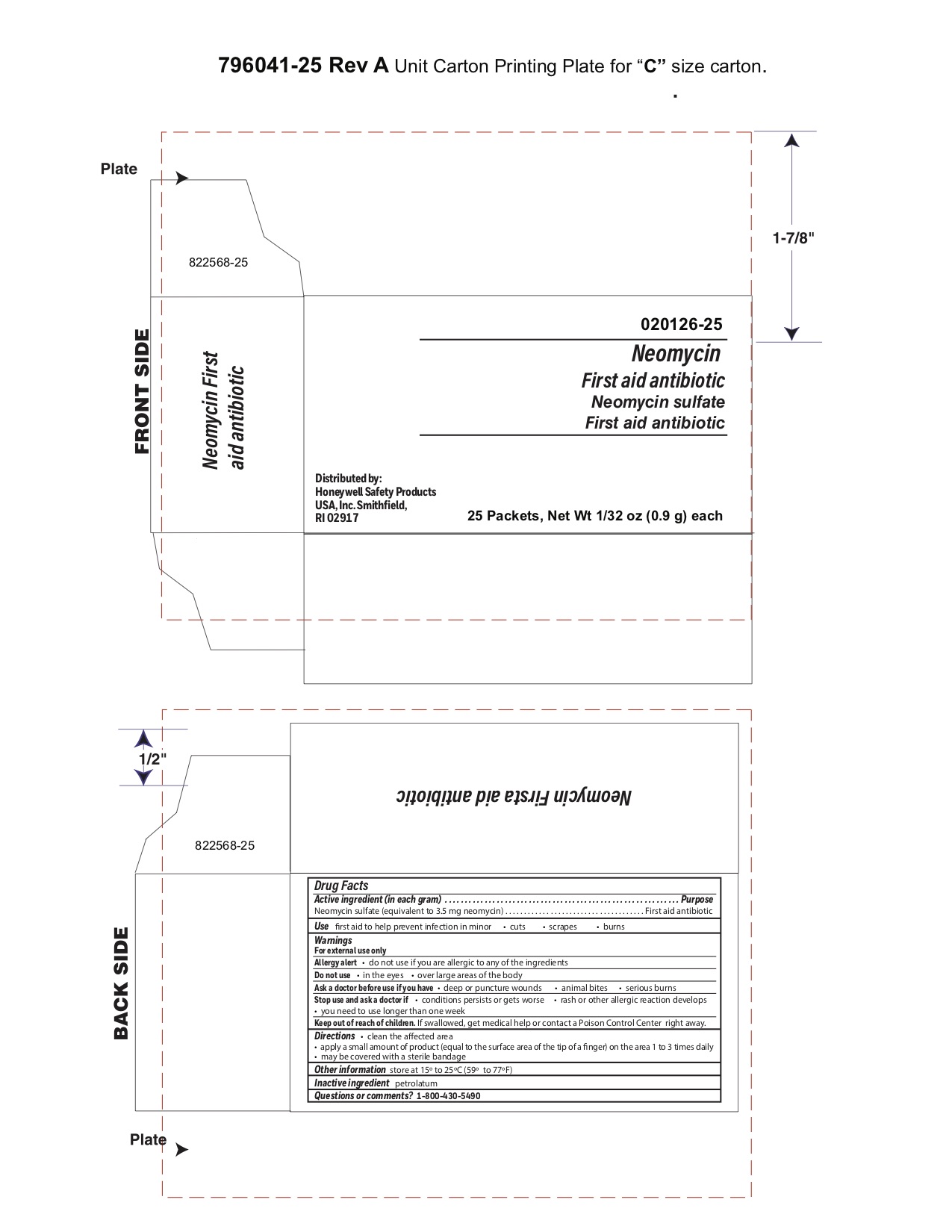
Neomycin
Warnings
For external use only
Stop use and ask a doctor if
- a rash or other allergic reaction develops
- you need to use longer than 1 week
Neomycin
Direction
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
BZK
Directions
- clean the affected area
- spray a small amount of this product on the area 1 to 3 times daily
- may be covered with a sterile bandage
- if bandaged, let dry first
BZK
Inactive ingredients
iazolidinyl urea, edetate disodium, glycerin, hypromellose, methylparaben, octoxynol 9, propylene glycol, propylparaben, trolamine, water
4263 Kit Contnets
SF00000637
1 NEOMYCIN ANTIBIOTIC 10 PER
1 GAUZE BANDAGE, 4" X 6 YD
1 TWEEZER PLASTICS 4"
1 ABD COMBINE PAD 5" X 9"
1 CPR FILTERSHIELD 77-100
1 1 OZ, BUFF EYEWASH
1 SCISSOR BDGE 4" RED PLS HDL
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
10 BZK ANTISEPTIC WIPE, BULK
2 PR LRG NITRILE GLVES ZIP BAG
1 TAPE ADHESIVE 1/2 X 2.5 125133
1 KIT, PP 16 UNIT FA
1 LABL INSTR FA REV A
1 TRI BNDG NON WOVEN 40"X40"X56"
1 COLD PACK UNIT 4"X6" BULK
2 EYE PADS STD OVAL STERILE
5 GAUZE PADS 4"X4" 12PLY
20 PLASTIC BANDAGE 3/4" X 3"
20 PLASTIC BANDAGE 1" X 3"
5 AYPANAL BULK 2/PK