Keep out of reach of children
if swallowed, get medical help of contact a Poison Control Center right away.
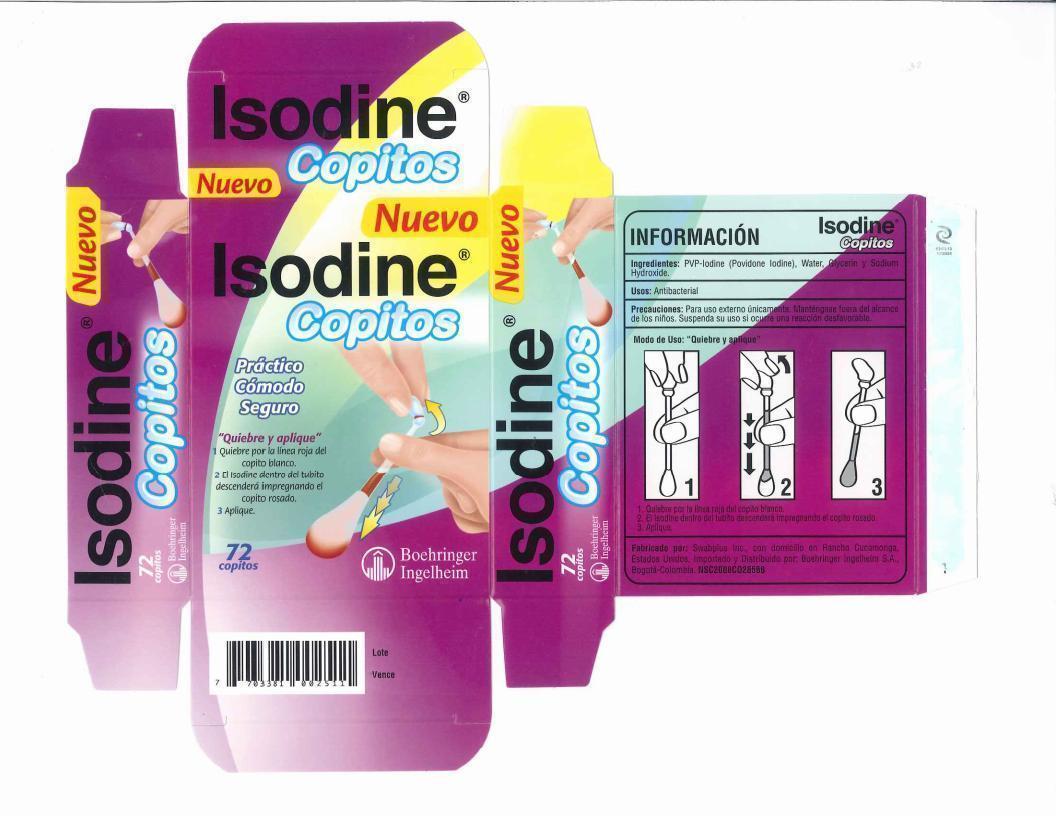
Directions
- Hold the swab vertically, with the color ring tip upwards, Hold n the center of the tube with one hand at the color ring with the other.
- Gently snap the tip with the color ring, Formula inside the tube flows down and fills the opposite tip.
- Apply the product to the affected area.
- Discard swab after use.
Warnings
For external use only. Keep out of eyes and ear canal. Non-Sterile. Discontinue use if irritation.
Do not use. If you are sensitive to Iodine. Longer than one week unless directed by a doctor.
Stop use and ask a doctor. If you have deep or puncture wounds, or serious burns. redness, irritation, swelling or pain continue or increas., infedtion occurs.
Administration
For adults and children 2 and older: for minor wounds, burns and infections, apply directly to affected area. May be covered with a bandage.
Children under 2 years of age: Do not use, consult a doctor.
Other information: Store art room temperature, Avoid direct sunlight, excessive heat and moisture.