DESCRIPTION
Pentamidine isethionate for injection, an anti-protozoal agent, is a sterile, nonpyrogenic, lyophilized product. After reconstitution, it should be administered by intramuscular (IM) or intravenous (IV) routes (see DOSAGE AND ADMINISTRATION). Pentamidine isethionate is a white crystalline powder soluble in water and glycerin, slightly soluble in alcohol and insoluble in ether, acetone, and chloroform. It is chemically designated as 4,4-[1,5-pentanediylbis(oxy)]bis-benzenecarboximidamid with the following structural formula:
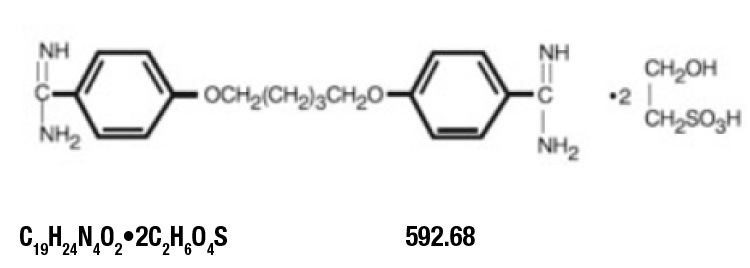
Each vial contains: Pentamidine isethionate . . . . . . . . . . . . . 300 mg
CLINICAL PHARMACOLOGY
Pentamidine isethionate, an aromatic diamidine, is known to have activity against Pneumocystis carinii. The mode of action of pentamidine is not fully understood. In vitro studies indicate that the drug interferes with protozoal nuclear metabolism by inhibition of DNA, RNA, phospholipid and protein synthesis.
Pharmacokinetics
Pharmacokinetic parameters following the administration of 4 mg/kg pentamidine isethionate as a single two-hour intravenous infusion or after a single intramuscular injection to 12 patients with AIDS are presented in the following table:
| Mean ± SD |
C max ng/mL |
Clearance L/h |
Half-life hours |
Vdss L |
Concentration ng/mL | |
| 8 hour | 24 hour | |||||
|
2 hour I.V. infusion 4 mg/kg (N=6) | 612 ± 371 | 248 ± 91 | 6.4 ± 1.3 | 821 ± 535 | 19.3 ± 16.9 | 2.9 ± 1.4 |
|
I.M. 4 mg/kg (N=6) | 209 ± 48 | 305 ± 81 | 9.4 ± 2.0 | 2724 ± 1066 | 22.9 ± 8.0 | 6.6 ± 3.5 |
In seven patients treated with daily IM doses of pentamidine at 4 mg/kg for 10 to 12 days, plasma concentrations were between 300 to 500 ng/mL. The concentrations did not appreciably change with time after injection or from day to day. Higher plasma concentrations were encountered in patients with an elevated blood urea nitrogen. The patients continued to excrete decreasing amounts of pentamidine in urine up to 6 to 8 weeks after cessation of the treatment.
Following multiple intravenous administration of pentamidine isethionate (3.7 to 4 mg/kg/day infused over 4 hours) to 6 patients with AIDS being treated for PCP, the pharmacokinetic parameters obtained on Days 1,4 and 7 are summarized in the following table:
| Mean ± SD |
C max * ng/mL |
C min * ng/mL |
Clearance mL/min |
Renal Clearance mL/min/1.73 m 2 |
Creatinine Clearance mL/min/1.73 m 2 |
| Day 1 | 175.3 ± 54 | --- | 5737 ± 1878 | 269 ± 149 | 97 ± 12 |
| Day 4 | 210.9 ± 80 | 17.6 ± 9.5 | 3350 ± 1944 | 214 ± 145 | 93 ± 17 |
| Day 7 | 256.7 ± 89 | 40.8 ± 16.1 | 1989 ± 566 | 134 ± 60 | 69 ± 17 |
*derived from Lidman
Compared to the mean AUC on Day 1, AUC on Day 4 and Day 7 were about 2 and 3 fold higher, respectively, suggesting that steady state was not achieved by Day 7 of dosing.
In other published reports of pharmacokinetics of pentamidine following daily intravenous doses of 2 to 4 mg/kg/day, clearance ranged from 30 to 40 mL/min/kg and Vdss ranged from 200 to 400 L/kg. Reported values for terminal half-lives of 2.8 to 12 days is suggestive of a deep peripheral compartment. In the urine, up to 12% of the administered dose has been recovered during a dosing interval as unchanged pentamidine.
Tissue distribution was studied in mice given a single intraperitoneal injection of pentamidine at 10 mg/kg. The concentration was highest in the kidneys followed by the liver. In mice, pentamidine was excreted unchanged, primarily via the kidneys with some elimination in the feces. The ratio of amounts excreted in the urine and feces (4:1) was constant over the period of study.
Tissue distribution has also been studied in normal and in renally impaired dogs (N = 3 each) given 13 mg/kg of pentamidine IV, in 2 doses separated by five weeks. The concentration of pentamidine was highest in the liver followed by kidneys and lungs. Pentamidine was concentrated in these organs approximately 70 to 1000 times that of the peak serum concentration. Similar findings were reported in normal and in renally impaired dogs (N = 2 each) given 97.5 mg/kg of pentamidine IV, in 15 daily doses. After repeated doses, the organs showed a further 3 to 7 fold accumulation while serum concentrations remained unchanged.
INDICATIONS AND USAGE
Pentamidine isethionate for injection is indicated for the treatment of pneumonia due to Pneumocystis carinii.
CONTRAINDICATIONS
Contraindicated in patients with a history of hypersensitivity to pentamidine isethionate.
WARNINGS
Fatalities due to severe hypotension, hypoglycemia, acute pancreatitis and cardiac arrhythmias have been reported in patients treated with pentamidine isethionate, both by the IM and IV routes. Severe hypotension may result after a single IM or IV dose and is more likely with rapid IV administration (see PRECAUTIONS). The administration of the drug should, therefore, be limited to the patients in whom Pneumocystis carinii has been demonstrated. Patients should be closely monitored for the development of serious adverse reactions (see PRECAUTIONS and ADVERSE REACTIONS).
Extravasations have been reported which, in some instances, proceeded to ulceration, tissue necrosis and/or sloughing at the injection site. While not common, surgical debridement and skin grafting has been necessary in some of these cases; long-term sequelae have been reported. Prevention is the most effective means of limiting the severity of extravasation. The intravenous needle or catheter must be properly positioned and closely observed throughout the period of pentamidine isethionate administration. If extravasation occurs, the injection should be discontinued immediately and restarted in another vein. Because there are no known local treatment measures which have proven to be useful, management of the extravasation should be symptomatic.
PRECAUTIONS
General
Pentamidine isethionate should be used with caution in patients with hypertension, hypotension, ventricular tachycardia, hypoglycemia, hyperglycemia, hypocalcemia, pancreatitis, leukopenia, thrombocytopenia, anemia, hepatic or renal dysfunction and Stevens-Johnson syndrome.
Patients may develop sudden, severe hypotension after a single dose of pentamidine isethionate, whether given IV or IM. Therefore, patients receiving the drug should be lying down and the blood pressure should be monitored closely during administration of the drug and several times thereafter until the blood pressure is stable. Equipment for emergency resuscitation should be readily available. If pentamidine isethionate is administered IV, it should be infused over a period of 60 to 120 minutes.
Pentamidine isethionate-induced hypoglycemia has been associated with pancreatic islet cell necrosis and inappropriately high plasma insulin concentrations. Hyperglycemia and diabetes mellitus, with or without preceding hypoglycemia, have also occurred, sometimes several months after therapy with pentamidine isethionate. Therefore, blood glucose levels should be monitored daily during therapy with pentamidine isethionate, and several times thereafter.
Renal and Hepatic Impairment
The efficacy or safety of alternative pentamidine isethionate for injection dosing protocols have not been established for patients with impaired renal or hepatic function.
Laboratory Tests
The following tests should be carried out before, during and after therapy:
a) Daily blood urea nitrogen and serum creatinine determinations.
b) Daily blood glucose determinations.
c) Complete blood count and platelet count.
d) Liver function test, including serum bilirubin, alkaline phosphatase, AST (SGOT), and ALT (SGPT).
e) Serum calcium determinations.
f) Electrocardiograms.
Drug Interactions
No drug interaction studies with pentamidine isethionate for injection have been conducted.
Because the nephrotoxic effects may be additive, the concomitant or sequential use of pentamidine isethionate and other nephrotoxic drugs such as aminoglycosides, amphotericin B, cisplatin, foscarnet, or vancomycin should be closely monitored and avoided, if possible.
Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been conducted to evaluate the potential of pentamidine isethionate as a carcinogen, mutagen, or cause of impaired fertility.
Pregnancy-Pregnancy Category C
Animal reproduction studies have not been conducted with pentamidine isethionate. It is also not known whether pentamidine isethionate can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Pentamidine isethionate should not be given to a pregnant woman unless the potential benefits are judged to outweigh the unknown risks.
Nursing Mothers
It is not known whether pentamidine isethionate is excreted in human milk. Because of the potential for serious adverse reactions in nursing infants from pentamidine isethionate, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. Because many drugs are excreted in human milk, pentamidine isethionate should not be given to a nursing mother unless the potential benefits are judged to outweigh the unknown risks.
Pediatric Use
Intravenous and intramuscular pentamidine has been described as an effective treatment for Pneumocystis carinii pneumonia (PCP) in immunocompromised pediatric patients beyond 4 months of age. The efficacy and safety profiles in these pediatric patients were similar to those observed in adult patients (See DOSAGE AND ADMINISTRATIONand OVERDOSAGE) .
ADVERSE REACTIONS
CAUTION: Fatalities due to severe hypotension, hypoglycemia, acute pancreatitis and cardiac arrhythmias have been reported in patients treated with pentamidine isethionate, both by the IM and IV routes. Nephrotoxic events (increased creatinine, impaired renal function, azotemia, and renal failure) are common with the parenteral administration of pentamidine isethionate. The administration of the drug should, therefore, be limited to the patients in whom Pneumocystis carinii has been demonstrated.
The most frequently reported spontaneous adverse events (1 to 30%) reported in clinical trials, regardless of their relation to pentamidine isethionate therapy were as follows (n=424):
| Cardiovascular: | |
| -Hypotension | 5.0% |
| Gastrointestinal: | |
| -Anorexia/Nausea | 5.9% |
| Hematologic: | |
| -Anemia | 1.2% |
| -Leukopenia | 10.4% |
| -Thrombocytopenia | 2.6% |
| Hepatic: | |
| -Elevated liver function tests | 8.7% |
| Metabolic: | |
| -Hypoglycemia | 5.9% |
| Neurologic: | |
| -Confusion/hallucinations | 1.7% |
| Skin: | |
| -Sterile abscess and/or necrosis, pain, or induration at the site of IM injection | 11.1% |
| -Rash | 3.3% |
| Special Senses: | |
| -Bad taste | 1.7% |
| Urogenital: | |
| -Azotemia | 8.5% |
| -Elevated serum creatinine | 23.6% |
| -Elevated blood urea nitrogen | 6.6% |
| -Impaired renal function | 28.8% |
Adverse events with a frequency of less than 1% incidence were as follows (No causal relationship to treatment has been established for these adverse events):
|
Body as a whole: |
Allergic reaction (i.e. urticaria, itching, rash), anaphylaxis, arthralgia, chills, extrapulmonary pneumocystosis, headache, night sweats, and Stevens-Johnson syndrome. |
|
Cardiovascular: |
Abnormal ST segment of electrocardiogram, cardiac arrhythmias, cerebrovascular accident, hypertension, palpitations, phlebitis, syncope, tachycardia, vasodilatation, vasculitis and ventricular tachycardia. |
|
Gastrointestinal: |
Abdominal pain, diarrhea, dry mouth, dyspepsia, hematochezia, hypersalivation, melena, pancreatitis, splenomegaly, and vomiting. |
|
Hematological: |
Defibrination, eosinophilia, neutropenia, pancytopenia, and prolonged clotting time. |
|
Hepatic: |
Hepatic dysfunction, hepatitis and hepatomegaly |
|
Metabolic: |
Hyperglycemia, hyperkalemia, hypocalcemia, and hypomagnesemia. |
|
Neurological: |
Anxiety, confusion, depression, dizziness, drowsiness, emotional lability, hypesthesia, insomnia, memory loss, neuropathy, nervousness, neuralgia, paranoia, paresthesia, peripheral neuropathy, seizure, tremors, unsteady gait, and vertigo. |
|
Respiratory system: |
Asthma, bronchitis, bronchospasm, chest congestion, chest tightness, coryza, cyanosis, eosinophilic or interstitial pneumonitis, gagging, hemoptysis, hyperventilation, laryngitis, laryngospasm, non-specific lung disorder, nasal congestion, pleuritis, pneumothorax, rales, rhinitis, shortness of breath, and tachypnea. |
|
Skin: |
Desquamation, dry and breaking hair, dry skin, erythema, dermatitis, pruritus, rash, and urticaria. |
|
Special senses: |
Blepharitis, blurred vision, conjunctivitis, contact lens discomfort, eye pain or discomfort, loss of hearing, loss of taste, and loss of smell. |
|
Urogenital: |
Flank pain, hematuria, incontinence, nephritis, renal dysfunction and renal failure. |
From post-marketing clinical experience with pentamidine isethionate, the following adverse events have been reported: cough, diabetes mellitus/ketoacidosis, dyspnea, infiltration (extravasation–see WARNINGS), and torsades de pointes.
To report SUSPECTED ADVERSE REACTIONS, contact XGen Pharmaceuticals DJB, Inc. at 1-866-390-4411 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
OVERDOSAGE
A 17 month old infant inadvertently received 1600 mg of intravenous pentamidine isethionate which was followed by renal and hepatic function impairment, hypotension and cardiopulmonary arrest. Treatment included cardiopulmonary resuscitation, epinephrine, atropine and intubation. In addition, a four hour course of charcoal hemoperfusion was accompanied by reduction of pentamidine serum concentration and stabilization of the patient’s condition. The patient recovered from these adverse events, but later died due to an unknown cause. 1
DOSAGE AND ADMINISTRATION
CAUTION:DO NOT USE SODIUM CHLORIDE INJECTION, USP FOR INITIAL RECONSTITUTION BECAUSE PRECIPITATION WILL OCCUR. Pentamidine isethionate should be administered IM or IV only. The recommended regimen for adults and pediatric patients beyond 4 months of age is 4 mg/kg once a day for 14 to 21 days. Therapy for longer than 21 days with pentamidine isethionate has also been used but may be associated with increased toxicity.
Intramuscular Injection
The contents of one vial (300 mg) should be dissolved in 3 mL of Sterile Water for Injection, USP at 22° to 30°C (72° to 86°F). The calculated daily dose should then be withdrawn and administered by deep IM injection.
Intravenous Injection
The contents of one vial (300 mg) should first be dissolved in 3 to 5 mL of Sterile Water for Injection, USP, or 5% Dextrose Injection, USP at 22°to 30°C (72° to 86°F). The calculated dose of pentamidine isethionate should then be withdrawn and diluted further in 50 to 250 mL of 5% Dextrose Injection, USP.
The diluted IV solutions containing pentamidine isethionate should be infused over a period of 60 to 120 minutes.
Aseptic technique should be employed in preparation of all solutions. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit.
Stability
After reconstitution with sterile water, the pentamidine isethionate solution is stable for 48 hours in the original vial at room temperature if protected from light. To avoid crystallization,store at 22° to 30°C (72° to 86°F). Intravenous infusion solutions of pentamidine isethionate at 1 mg/mL and 2.5 mg/mL prepared in 5% Dextrose Injection, USP are stable at room temperature for up to 24 hours.
Intravenous (IV) solutions of pentamidine isethionate have been shown to be incompatible with fluconazole and foscarnet sodium. IV solutions of pentamidine isethionate have been shown to be compatible with IV solutions of zidovudine (AZT) and diltiazem hydrochloride.
HOW SUPPLIED
| NDC No. |
| Pentamidine isethionate for injection 300 mg, lyophilized product in Single-dose Vials (NDC 39822-3050-1), packages of 10 (NDC 39822-3050-2). |
Store dry product at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from light.
Preservative Free. Discard unused portion.
REFERENCE
- Watts RG; Conte JE, Jr.; Zurlinden E; Waldo FB: Effect of charcoal hemoperfusion on clearance of pentamidine isethionate after accidental overdose. J Toxicol Clin Toxicol 1997;35:89-92.
Manufactured for:
XGen Pharmaceuticals DJB, Inc.
Big Flats, NY 14814
Revised: March 2022
PENJ-PI-06
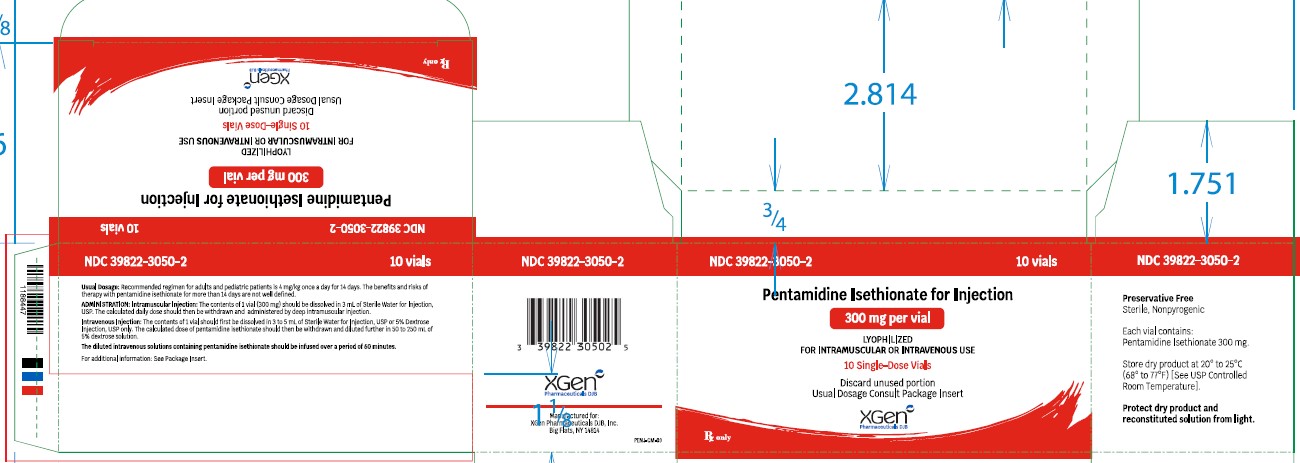
[PACKAGE LABEL.PRINCIPAL DISPLAY PANEL]
PACKAGE LABEL - PRINCIPAL DISPLAY – PENTAMIDINE ISETHIONATE FOR INJECTION 300 mg Single Dose Vial Label
NDC 39822-3050-1
Pentamidine Isethionate for Injection)
300 mg
Lyophilized
For IM or IV Use
Single-Dose Vial
Rx only

PACKAGE LABEL - PRINCIPAL DISPLAY –PENTAMIDINE ISETHIONATE FOR INJECTION 300 mg Single Dose Vial 10 Pack Carton
NDC 39822-3050-2
Pentamidine Isethionate for Injection)
300 mg
Lyophilized
For IM or IV Use
Single-Dose Vial
Rx only