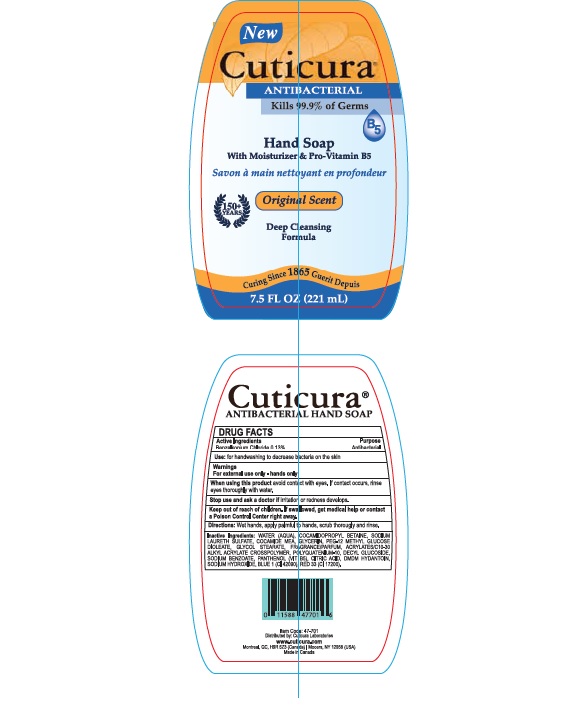
When using this product avoid contact with eyes. If contact occurs, rinse eyes thoroughly with water
Stop use and ask a doctor if
- irritaion or redness develops
- condition persists for more than 72 hours
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away
Inactive Ingredients: Water (Aqua),Acrylates/C10-30 Alkyl Acrylate crosspolymer, Citrtic Acid, Cocamidopropyl Betaine,Cocamide MEA,Decyl Glucoside, DMDM Hydantoin,Sodium Benzoate, Sodium Laureth Sulfate, Sodium Chloride, Sodium Hydroxide, Glycerin, Fragrance,Panthenol (Parfum),PEG-12 Methyl Glucose Dioleate, Polyquatenium 10, Tetrasodium EDTA, Blue 1, Red# 4 (Cl 14700)