Uses
- relieves occasional constipation (irregularity)
- generally produces a bowel movement in 12 to 72 hours
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel movements that lasts over 2 weeks
Directions
Take only by mouth. Doses may be taken as a single daily dose or in divided doses.
|
adults and children 12 years and over |
take 1 to 3 softgels daily |
|
children 2 to under 12 years of age |
take 1 softgel daily |
|
children under 2 years |
ask a doctor |
Other information
- each softgel contains: sodium 5 mg
- VERY LOW SODIUM
- store at room temperature 15°-30°C (59°-86°F) and avoid excessive heat
Inactive ingredients
Black ink, citric acid, D&C red #33, FD&C blue #1, FD&C red #40, FD&C yellow #6, gelatin, glycerin, polyethylene glycol, propylene glycol, sorbitol special and purified water
Manufactured by:
Humanwell PuraCap Pharmaceutical (Wuhan) Ltd.
Wuhan, Hubei
430206, China
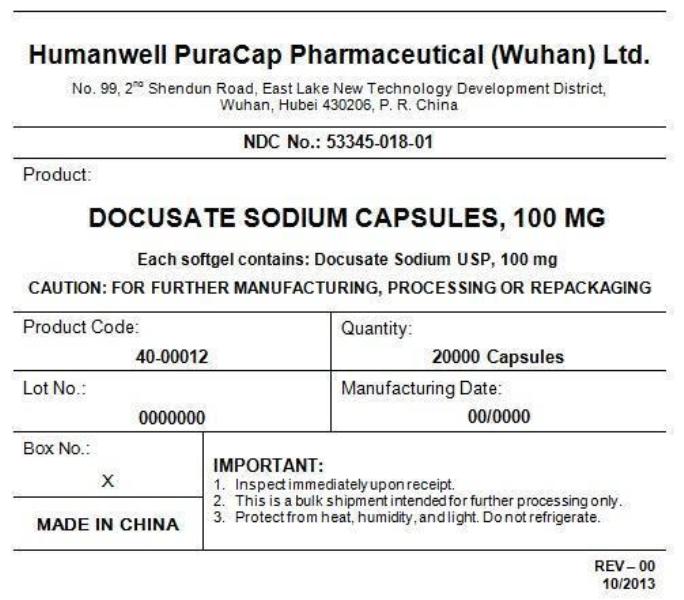
PRINCIPAL DISPLAY PANEL - Shipping Label
DOCUSATE SODIUM CAPSULES, 100 mg
Quantity : 20000 Capsules
NDC. No : 53345-018-01
IMPORTANT:
1.Inspect immediately upon receipt.
2.This is a bulk shipment, intended for further processing only.
3.Protect from heat, humidity, and light. Do not refrigerate.
CAUTION : FOR FURTHER MANUFACTURING, PROCESSING OR REPACKAGING
DOCUSATE SODIUM CAPSULES, 100 mg
Quantity : 15000 Capsules
NDC. No : 53345-018-02
IMPORTANT:
1.Inspect immediately upon receipt.
2.This is a bulk shipment, intended for further processing only.
3.Protect from heat, humidity, and light. Do not refrigerate.
4.Store at 15-30°C (59-86°F)
CAUTION : FOR FURTHER MANUFACTURING, PROCESSING OR REPACKAGING
