Eyewash
Uses
- for flushing the eye to remove loose foreign material, air pollutants or chlorinated water
Eyewash
Warnings
For external use only Obtain immediate medical treatment for all open wounds in or near eyes. To avoid contamination, do not touch tip of container to any surface. Do not reuse. Once opened, discard.
Do not use
- if solution changes color or becomes cloudy
- if you have open wounds in or near the eyes, get medical help right away.
Eyewash
Directions
- remove contacts before using
- twist top to remove
- flush the affected area as needed
- control rate of flow by pressure on the bottle
- if necessary, continue flushing with emergency eyewash or shower
Water Soluble 1st Aid Spray
Uses
for temporary relief of pain and itching and helps protect against infection in
- minor cuts and scrapes
- insect bites
- minor skin irritations
Water Soluble 1st Aid Spray
Warnings
For external use only
Flammable
- keep away from fire or flame
- contents under pressure
- do not puncture or incinerate container
- do not expose to temperature above 120 0 F
Do not use
- in the eyes or other mucous membranes
- in cases of serious burns
- in case of deep orpuncture wounds
- for a prolonged period of time
- on large portion of the body
Water Soluble 1st Aid Spray
Directions
- clean the affected area
- shake can well before using
- hold 4 - 6 inches from surface and spray area until wet
- may be covered with a sterile bandage. If bandaged, let dry first
- for adult institutional use only
- not intended for use on children
Water Soluble 1st Aid pray
Other information
- avoid inhaling
- use only as directed
- intentional misuse by deliberately concentrating and inhaling the contents may be harmful or fatal
Burn Relief Water Soluble
Active ingredients
Benzethonium chloride 0.2% w/w
Benzocaine 10% w/w
Menthol 0.33% w/w
Burn Relief Water Soluble
Uses
for the temporary relief of pain and itching and helps protect against infection in:
- minor cuts and scrapes
- burns
- sunburn
- insect bites
- minor skin irritations
Burn Relief Water Soluble
Warnings
For external use only
Flammable keep away from fire or flame
- contents under pressure
- do not puncture or incinerate container
- do not expose to temperatures above 120 0 F
Do not use
- in or near the eyes or other mucous membranes
- in case of serious burns
- in case of deep or puncture wounds
- for prolonged period of time
- on large portion of the body
Burn Relief Water Soluble
Directions
- clean the affected area
- shake can well before using
- hold 4 - 6 inches from surface and spray area until wet
- may be covered with a sterile bandage, if bandaged let dry first
- for adult institutional use only
- not intended for use on children
Burn Relief Water Soluble
Other information
- avoid inhaling
- use only as directed
- intentional misuse by deliberately concentrating or inhaling the contents may be harmful or fatal
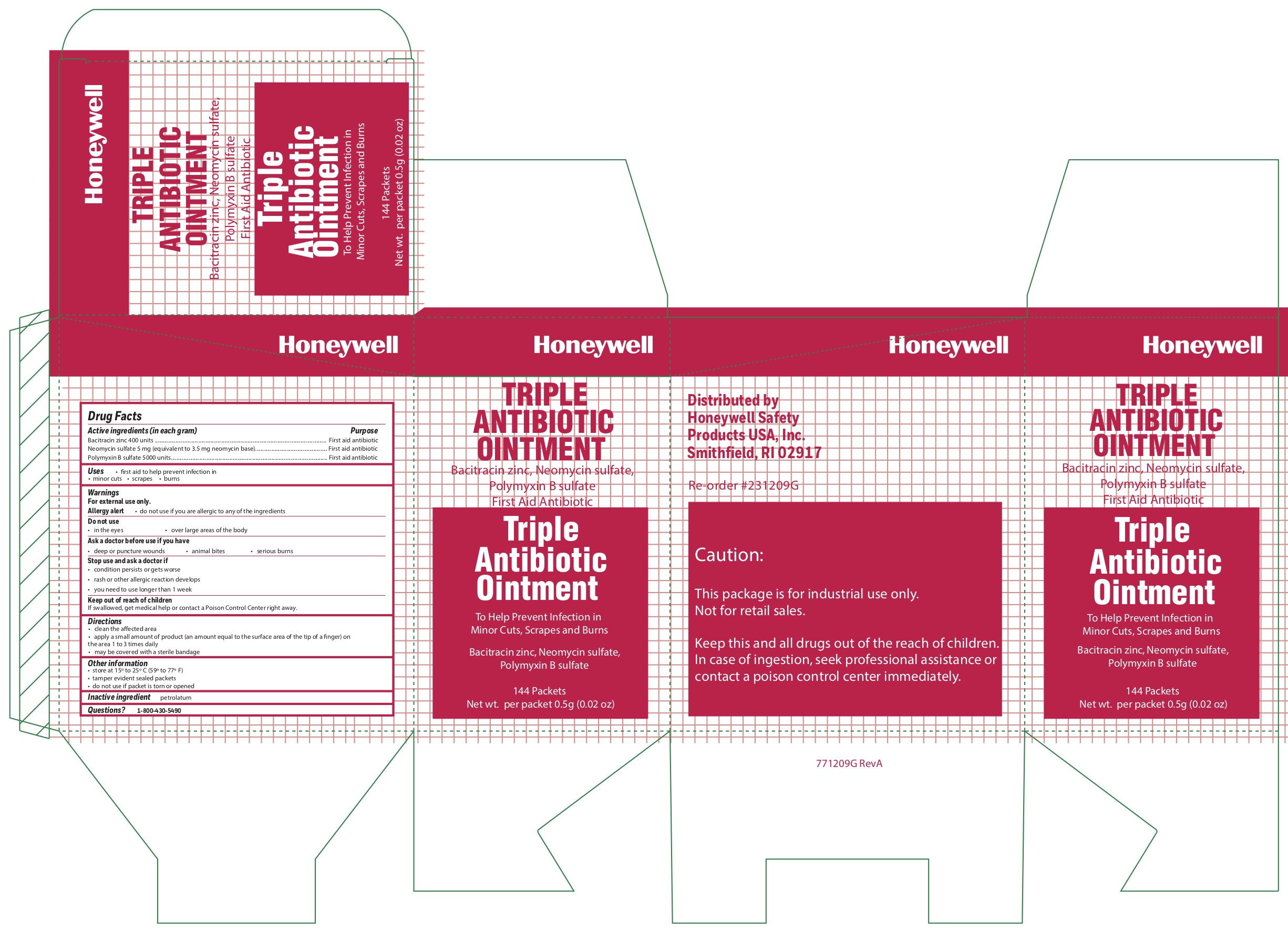
Triple
Active ingredients
Bacitracin zinc 400 units
Neomycin sulfate (5 mg equivalent to 3.5 mg Neomycin base)
Polymyxin B sulfate 5000 units
Triple
Warnings
For external use only
Allergy alert do not use if you are allergic to any of the ingredients
Triple
Directions
- clean the affected area
- apply a small amount of the product (an amount equal to the surface area of the tip of a finger) on the area 1 to 3 times daily
- may be covered with a sterile bandage
Triple
Other information
- store at 15 0 to 25 0 C (59 0 to 77 0 F)
- tamper evident sealed packets
- do not use if packet is torn or opened
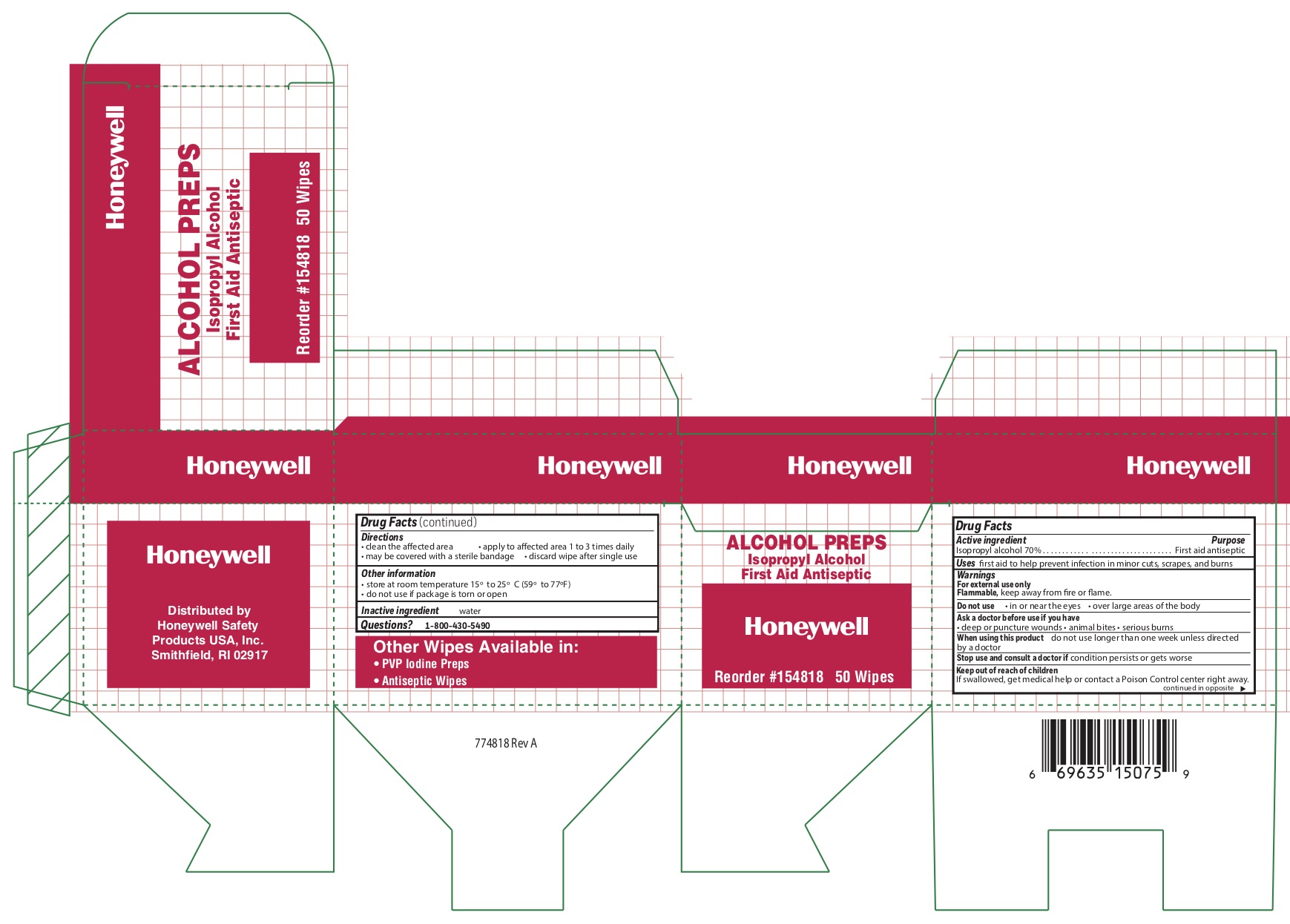
Alcohol
Directions
- clean the affected area
- apply wipe to affected area 1 to 3 times daily
- may be covered with a sterile bandage
- discard wipe after single use
BZK
Warnings
For external use only
Do not use
- in the eyes or over large areas of the body
- on mucous membranes
- on irritated skin
- in case of deep puncture wounds, animal bites or serious burns, consult a doctor
- longer than 1 week unless directed by a doctor
BZK
Other information
- store at room temperature 15 0 to 30 0 C (59 0 - 86 0 F)
- do not reuse towelette
4242
68400LWS KIT CONTENTS
1 3/4 X 3 PLAS 100/BOX
1 1X3 PLASTIC 100/BOX
1 WOVEN 2" X 3" 25/BOX
1 FINGERTIP "T" WOVEN 40/BOX
1 1X3 WOVEN SING 50/BOX
1 SWIFT KNUCKLE 40/BX
1 ELASTIC TAPE 1" X 5YD
1 FORCEPS POINTED METAL
1 O/H TAPE ADHESIVE TRI-CUT
1 FIRST AID GUIDE ASHI
6 GAUZE CLEAN-WRAP BDGE N/S 2"
1 BLOODSTOPPER
1 NON ADHERENT PADS 2"X3" 50'S
2 GZE PADS STERILE 2"X 2" 25'S
1 GZE PADS STERILE 4"X 4" 25'S
1 COTTON TIPS 100 PER VIAL
1 ANTISEPTIC WIPES BZK CHL 20'S
1 FIRST AID SPRAY AEROSOL 3 OZ
1 ALCOHOL WIPES 50'S
1 BURN SPRAY 3 OZ
1 TRIPLE BIOTIC .5 GRAM PKT 20
1 COLD PACK 5"X9" BOXED
1 4OZ BFS EYEWASH TRILINGUAL BOTTLE
1 SCISSOR BDGE 4" RED PLS HDL
1 400 EMPTY KIT BLANK
1 POCKET INSERT RED #400 KIT 5R
1 TONGUE BLADES SR WRAPPED 6'S
1 LBL STOCK 6-3/8"X4"
1 LBL STOCK 4"X2-7/8"
2 PR LRG NITRILE GLVES ZIP BAG
2 TRI BNDG NON WOVEN 40"X40"X56"
1 RED BIO BAGS 2/BX