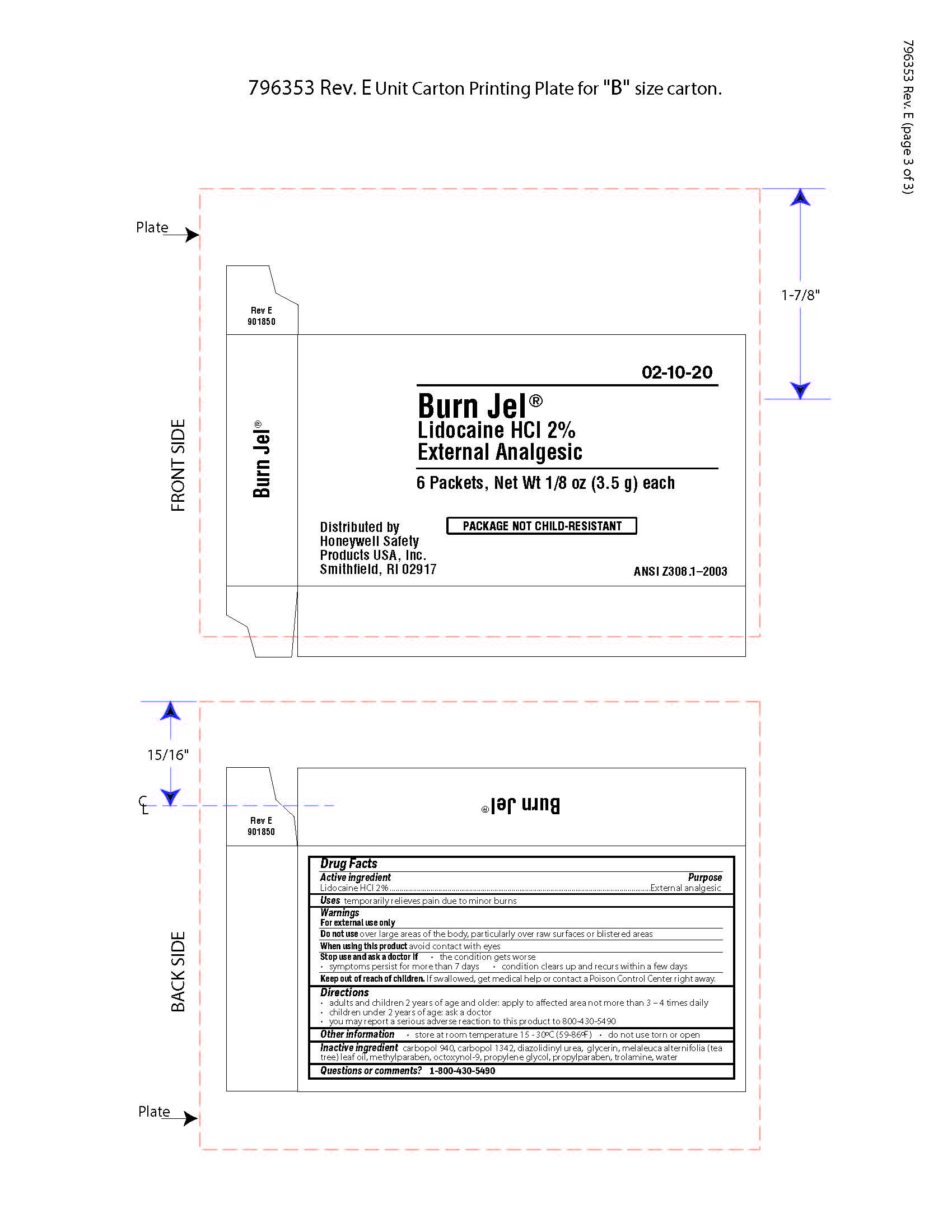
Burn Jel
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
Burn Jel
Directions
- adults and children 2 years of age and older; apply to affected area not more than 3 to 4 times daily
- children under 2 years of age: ask a doctor
- you may report a serious reaction to this product to 800-430-5490
Burn Jel
Inactive ingredients
carbopol 940, carbopol 1342, diazolidinyl urea, glycerin, melaleuca alternifolia (tea tree) leaf oil, methylparaben, octoxynol-9, propylene glycol, propylparaben, trolamine, water
PVP Wipe
Keep out of reach of children
If swallowed, get medical help or contact a Poison Control Center right away.
PVP Wipe
Directions
- clean the affected area
- apply1 to 3 times daily
- may be covered with a sterile bandage
- if bandaged, let dry first
- discard wipe after single use
PVP Wipe
Other information
- do not use on individuals who are allergic or sensitive to iodine
- store at controlled temperature 59-86ºF (15-30ºC)
- do not use if pouch is open or torn
4236
6824COS Kit Contents
1 EYE DRESS PKT W/4 ADH STRIPS
6 TRIANGULAR BDG, NON-STERILE
2 GAUZE COMPRESS, 1728 SQ IN 1
1 INSTANT COLD PACK 4" X 6"
3 ADHESIVE BDG,PLSTIC,1"X3"16PER
1 BURN JEL 1/8 OZ, 6 PER
2 PVP IODINE WIPES 10 PER
1 NITRILE GLOVES 2PR BBP
1 FIRST AID GUIDE ASHI
2 BANDAGE COMP 4" W/TELFA PAD 1
LBL STOCK 6-3/8"X4"
LBL STOCK 4"X2-7/8"
1 LBL STOCK 3"x1-7/8"
1 KIT STL 24 UN WHITE 01
1 WOVEN KNUCKLE 8'S
1 FINGERTIP "T" 8/BX
1 GAUZE PADS 3"X3" 4/BX
1 SCISSOR & FORCEP 1 EA